��Ŀ����
 ijͬѧΪ�ⶨij����ʯ��̼��ƣ����裺����ʯ�е����ʲ������ᷴӦ��������������ȡ�ķ���Ʒ�ֱ��ϡ���ᷴӦ����ʵ�����ݼ�¼���£�
ijͬѧΪ�ⶨij����ʯ��̼��ƣ����裺����ʯ�е����ʲ������ᷴӦ��������������ȡ�ķ���Ʒ�ֱ��ϡ���ᷴӦ����ʵ�����ݼ�¼���£�| ��Ʒ | ��1�� | ��2�� | ��3�� | ��4�� |
| ȡ��Ʒ������g�� | 10.0 | 10.0 | 10.0 | 10.0 |
| ȡϡ����������g�� | 25.0 | 50.0 | 75.0 | 100.0 |
| ��������������g�� | 1.1 | X | 3.3 | 3.3 |
��1�������������ڵ�1�β�õ������У�
��2��������X=
��3���ô���ʯ��Ʒ��̼��Ƶ����������Ƕ��٣�
��4��������10.0g��Ʒ�м�ϡ�����������������������仯��ϵ��ʾ��ͼ��
��������1�����ݵ�һ���м���25.0gϡ����ų�1.1g������̼���壬�����ݼ���75.0gϡ����ų�3.3g������̼���壬˵��25.0gϡ������ȫ��Ӧ������Ʒ��̼�����ʣ����н��
��2������ÿ25.0gϡ������ȫ��Ӧ�ͷų�1.1g������н��
��3���������ɶ�����̼�����������Ʒ��̼��Ƶ����������������ʯ��Ʒ��̼��Ƶ������������ɣ�
��4������10.0g��Ʒ����3.3g������̼�Լ���ȥ75.0gϡ������н��
��2������ÿ25.0gϡ������ȫ��Ӧ�ͷų�1.1g������н��
��3���������ɶ�����̼�����������Ʒ��̼��Ƶ����������������ʯ��Ʒ��̼��Ƶ������������ɣ�
��4������10.0g��Ʒ����3.3g������̼�Լ���ȥ75.0gϡ������н��
����⣺��1����һ���м���25.0gϡ����ų�1.1g������̼���壬�����ݼ���75.0gϡ����ų�3.3g������̼���壬˵��25.0gϡ������ȫ��Ӧ������Ʒ��̼�����ʣ�ࣻ���ϡ���
��2��ÿ25.0gϡ������ȫ��Ӧ�ͷų�1.1g���壬���Ե�2���м���50.0gϡ������ȫ��Ӧ�ų�2.2g���壻���2.2��
��3����ͼ��֪���ɶ�����̼������Ϊ3.3g
�����ʯ��Ʒ��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 3.3g
=
x=7.5g
�ô���ʯ��Ʒ��̼��Ƶ���������=
��100%=75%
�𣺸ô���ʯ��Ʒ��̼��Ƶ�����������75%��
��4������10.0g��Ʒ����3.3g������̼����ȥ75.0gϡ���ᣬ����10.0g��Ʒ�м�ϡ�����������������������仯��ϵ��ʾ��ͼΪ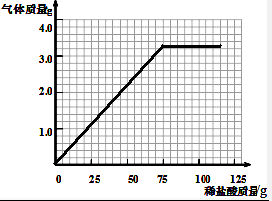 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
��2��ÿ25.0gϡ������ȫ��Ӧ�ͷų�1.1g���壬���Ե�2���м���50.0gϡ������ȫ��Ӧ�ų�2.2g���壻���2.2��
��3����ͼ��֪���ɶ�����̼������Ϊ3.3g
�����ʯ��Ʒ��̼��Ƶ�����Ϊx
CaCO3+2HCl�TCaCl2+H2O+CO2��
100 44
x 3.3g
| 100 |
| x |
| 44 |
| 3.3g |
x=7.5g
�ô���ʯ��Ʒ��̼��Ƶ���������=
| 7.5g |
| 10g |
�𣺸ô���ʯ��Ʒ��̼��Ƶ�����������75%��
��4������10.0g��Ʒ����3.3g������̼����ȥ75.0gϡ���ᣬ����10.0g��Ʒ�м�ϡ�����������������������仯��ϵ��ʾ��ͼΪ
 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�����������⿼���ۺϡ�ȫ�棬������ÿ�μ���ϡ������������������ı仯���Է�Ӧ���е���������жϣ����ǽ������Ļ���������ɺܺÿ���ѧ���������������������������漰�ķ���ʽ����϶࣬Ҫ����֪����ϸ�ķ�����

��ϰ��ϵ�д�
 ���Ͱ�ͨ��ĩ���ϵ�д�
���Ͱ�ͨ��ĩ���ϵ�д�
�����Ŀ
ijͬѧΪ�ⶨij����ʯ��̼��ƣ����裺����ʯ�е����ʲ������ᷴӦ��������������ȡ�ķ���Ʒ�ֱ��ϡ���ᷴӦ����ʵ�����ݼ�¼����:
|
��Ʒ |
��1�� |
��2�� |
��3�� |
��4�� |
|
ȡ��Ʒ������g�� |
10.0 |
10.0 |
10.0 |
10.0 |
|
ȡϡ����������g�� |
25.0 |
50.0 |
75.0 |
100.0 |
|
��������������g�� |
1.1 |
X |
3.3 |
3.3 |
����㣺
��1�������������ڵ�1�β�õ������У� �������ʣ���ȫ��Ӧ�ˣ�
��2��������X= g��
��3���ô���ʯ��Ʒ��̼��Ƶ����������Ƕ��٣�
��4��������10.0g��Ʒ�м�ϡ�����������������������仯��ϵ��ʾ��ͼ��