��Ŀ����
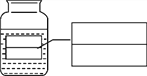
����Ŀ��С��ͬѧ��̽���������ʵ�ʵ�飨ͼ1�������˸Ľ�����ͼ2��ʾ����T����ͨ�ܵĴֲ�������̶�һ��ʪ��ķ�̪ɴ��������ش�ʵ���е��й����⡣

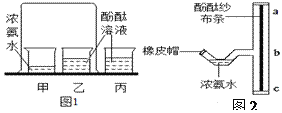
��1��ͼ2ʵ������____�����������ƣ���ȡ����Ũ��ˮ���������T����ͨ�ܵ�ϸ��ܴ���Ȼ������Ƥñ��չܿڡ�һ������۲쵽___���ba����bc������ɴ������ɺ�ɫ��
��2��ͼ1��װ�õ�������_____________�� ͼ1��ͼ2��ʵ�����֤������______����ͼ1ʵ����ȣ��Ľ���ͼ2ʵ����ŵ���_____________________��
��3������ͼ2��װ��Ũ��ˮ��ϸ��ܴ������ˮ�У��ɹ۲쵽ɴ�����������ʱ�____����족��������������۽ǶȽ��н��ͣ�_______��
���𰸡� ��ͷ�ιܣ�д���ιܡ�Ҳ�÷֣� ba �Ա�ʵ�� �ڲ��ϵ��˶� ��ʡҩƷ����ֹ��Ⱦ������ �� �¶�Խ�ͣ������˶�����Խ�������¶�Խ�ߣ������˶�����Խ��
����������1�����ݽ�ͷ�ιܿ�������ȡ������Һ����н�𣻸��ݰ������ܶȱȿ���С���н�𣻣�2�����ݶԱ����������𣻸��ݷ����Dz����˶��Ľ�𣻸���ʵ��װ�ò�ͬ����ȱ�㲻ͬ���н������3�������¶�Խ�ͣ������˶�����Խ���������1��ͼ2ʵ�����ý�ͷ�ι���ȡ����Ũ��ˮ���������T����ͨ�ܵ�ϸ��ܴ���Ȼ������Ƥñ��չܿڡ����ڰ������ܶȱȿ���С��һ����۲쵽ba��ɴ������ɺ�ɫ����2��ͼ1��װ�õ������ǶԱ�ʵ����ͼ1��ͼ2��ʵ��ͨ�����ձ��е���Һ����ba�α�죬����֤�������ڲ��ϵ��˶�����ͼ1ʵ����ȣ��Ľ���ͼ2ʵ����ŵ��ǽ�ʡҩƷ����ֹ��Ⱦ����������3������ͼ2��װ��Ũ��ˮ��ϸ��ܴ������ˮ�У��ɹ۲쵽ɴ�����������ʱ��������۽ǶȽ��н��ͣ��¶�Խ�ͣ������˶�����Խ�������¶�Խ�ߣ������˶�����Խ������

 �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�����Ŀ�������仯����Ӧ�ù㷺��
(һ)�����仯�����Ӧ��
����ͼ3ΪijС�����������ǽ���
(1)�ʵ����ø��ǽ�������______________(ѡ�����)��
A�����Ͳ� B��٪��֢ C��ƶѪ֢
(2)ͼ�����漰���IJ�����_________________(ѡ�����)��
A�������� B���ϳɲ��� C�����ϲ���
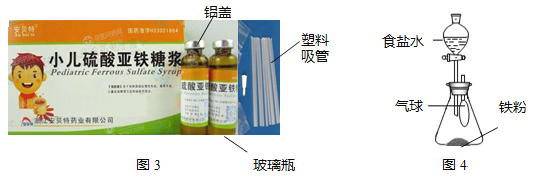
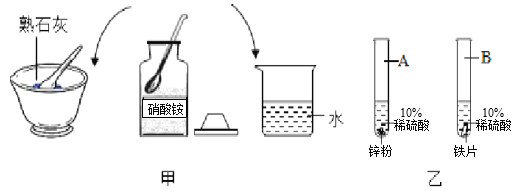
��ʳƷ������˫�����������ۡ�ʳ�εȣ���ͼ4Ϊ��ԭ��̽��ʵ�飺
(1)����ʳ��ˮ�رշ�Һ©��������һ��ʱ��������ϳ��ֺ�ɫ���壬����______(ѡ�������������������������С��)��
(2)ʳ��ˮ��������______��
(��)�����仯�����ʵ��
(1)������м�������ϴ�Ӽ�����ˮ�У���ֽ����Գ�ȥ���ۣ���������ϴ�Ӽ���______���á�
(2)ȡ�����Ĺ�����м����һ������ϡH2SO4(ˮԡ���ȿ�����50��80��)�����衢���á����ˡ�ϴ�ӣ�����������ϴ���ķ�����________��
(3)������������Һ�м��뱥��(NH4)2SO4��Һ��������Ũ����________�����˵Ȳ����õ�dz����ɫ������
���������ϣ���������Ԫ�ص�dz����ɫ�����У���������������[FeSO47H2O]��
����������茶���[FeSO4(NH4)2SO46H2O]��
(4)ȡһ����������Ʒ����������ˮ������������______��Һ���ȣ����Ѳ���������ͨ���̪��Һ����̪��Һ���______ɫ��˵���þ�������������茶��塣
(��)��������茶�����ȷֽ�ʵ��
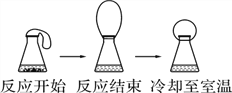
��ȤС���ȡ�˾�����Ʒ39.2g���ڿ�����Ա��ָ��������ͼװ�ý����ȷֽ�ʵ�顣
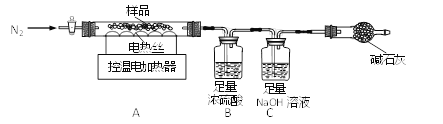
����������������������茶���(��Է�������Ϊ392)��100����ȫʧȥ�ᾧˮ��ʣ������500����ȫ�ֽ�Ϊ����ij�����SO2��SO3��NH3��H2O��
��Bװ����Ũ����ֻ������NH3��SO3��H2O��
(1)װ���ʵ��װ�ú���Ҫ________��
(2)����ǰ�����о�ͨ��N2��ֹͣ���Ⱥ����ͨN2��Ŀ���Ƿ�ֹ������________��
(3)���Ʋ�ͬ���¶ȶ�A�й�����ȣ����װ��B��C�е������仯���±���
�¶�/�� | ���� | 100 | 500 |
Bװ��/g | 200.00 | x | 228.00 |
Cװ��/g | 100.00 | 100.00 | 103.20 |
�ٱ�����x =________��
����������SO3������Ϊ________g������ij������Ļ�ѧʽΪ________��