��Ŀ����
��ѧ�����������ߣ�������������������ϢϢ��أ��������³��������ʣ�
��CO2 ��CH3COOH ��NaOH ��NaCl ��NaHCO3��C2H5OH ��SO2 ��kNO3 ��Ca��OH��2
��ѡ��������ʵ������գ�
��1����ɫֲ����й��������Ҫ���� ��
��2��ʳ���к��е��� ��
��3���׳��ռ���� ��
��4��ҽ������������������ˮ���� ��
��5�����Ƹ�㡢����θ������С�մ��� ��
��6������ę́�к��е��� ��
��7��������Ҫ������Ⱦ��֮һ���ǵ����������Ҫ���� ��
��8�����ڸ��Ϸ��ϵ��� ��
��9������������������ʯ���� ��
��CO2 ��CH3COOH ��NaOH ��NaCl ��NaHCO3��C2H5OH ��SO2 ��kNO3 ��Ca��OH��2
��ѡ��������ʵ������գ�
��1����ɫֲ����й��������Ҫ����
��2��ʳ���к��е���
��3���׳��ռ����
��4��ҽ������������������ˮ����
��5�����Ƹ�㡢����θ������С�մ���
��6������ę́�к��е���
��7��������Ҫ������Ⱦ��֮һ���ǵ����������Ҫ����
��8�����ڸ��Ϸ��ϵ���
��9������������������ʯ����
���㣺������̼����;,��������Ⱦ����Σ��,����������ʼ���;,����������Ժ���;,�����ε���;,�������ʵ����������,���顢�Ҵ��ȳ����л�������ʺ���;
ר�⣺���ʵ���������;
��������ɫֲ����й��������Ҫ���Ƕ�����̼��ˮ��CH3COOH�Ǵ������Ҫ�ɷ֣�NaOH�������ռNaCl��ҽ������������������ˮ���Ƶ���Ҫ�ɷ���C2H5OH��SO2���ڿ�����Ⱦ���������ڸ��Ϸʣ���ʯ�ҿ������ڸ�������������
����⣺��1����ɫֲ����й��������Ҫ���Ƕ�����̼��ˮ������٣�
��2��CH3COOH�Ǵ������Ҫ�ɷ֣�����ڣ�
��3��NaOH�������ռ����ۣ�
��4��NaCl��ҽ������������������ˮ������ܣ�
��5�����Ƹ�㡢����θ������С�մ���NaHCO3������ݣ�
��6���Ƶ���Ҫ�ɷ���C2H5OH�������
��7��SO2���ڿ�����Ⱦ����ǵ����������Ҫ���壬����ߣ�
��8��KNO3�к��м�Ԫ�غ͵�Ԫ�أ����ڸ��Ϸʣ�����ࣻ
��9����ʯ�Ҿ���Ca��OH��2�ͣ��������ڸ�����������������ᣮ
��2��CH3COOH�Ǵ������Ҫ�ɷ֣�����ڣ�
��3��NaOH�������ռ����ۣ�
��4��NaCl��ҽ������������������ˮ������ܣ�
��5�����Ƹ�㡢����θ������С�մ���NaHCO3������ݣ�
��6���Ƶ���Ҫ�ɷ���C2H5OH�������
��7��SO2���ڿ�����Ⱦ����ǵ����������Ҫ���壬����ߣ�
��8��KNO3�к��м�Ԫ�غ͵�Ԫ�أ����ڸ��Ϸʣ�����ࣻ
��9����ʯ�Ҿ���Ca��OH��2�ͣ��������ڸ�����������������ᣮ
���������⿼����л�ѧ�г����ļ������ʵ���;����Ŀ�Ƚϼ���ƽʱע�⽫��ѧ֪ʶ������������ϵ��һ����ô�����������ģ�

��ϰ��ϵ�д�
 ����ѧ��ţ��Ӣ��ϵ�д�
����ѧ��ţ��Ӣ��ϵ�д�
�����Ŀ
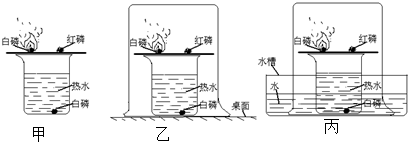
 ��1����ͼ��ʾ����������ˮ���ռ��õ���ɫ���壮�ݴˣ������ж�������������ص����������ǣ�
��1����ͼ��ʾ����������ˮ���ռ��õ���ɫ���壮�ݴˣ������ж�������������ص����������ǣ�