��Ŀ����
����Ŀ����Ҫ����60 g������������Ϊ5%���Ȼ�����Һ�������������ա�
��ʵ����̣�
��1������:��Ҫ�Ȼ��Ƶ�����Ϊ3g����Ҫˮ�����Ϊ_________mL��
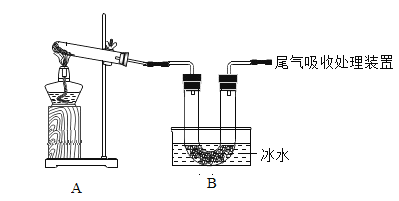
��2����ȡ�Ȼ���:�ڼ�����ƽ�����ƽ���˵������Ϸֱ����������ȵ�ֽƬ���ƶ��������̶���____________(ѡ��ס����ҡ�)ͼ��ʾ.
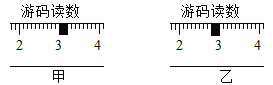
��3����ȡˮ:������Ͳ��ֱ�Ӽ�ˮ����ˮ�ӽ��̶�ʱ����_________������ˮ������̶ȡ�
��4���ܽ�:�ܽ�ʱ���ò����������Ŀ����___________��
��5��ת��:�������Һ����ָ�����������ϱ�ǩ��
��ʵ�鷴˼��
��6������ʵ���лᵼ��������Һ��������������ƫС����__________��
A�Ȼ��Ʒ�ĩ����
B����Ͳ��ȡˮʱ���Ӷ���
C������Һ���ձ���������������ˮ��ϴ
Dת�Ƽ���õ���Һʱ��������Һ�彦��
���𰸡�57 �� ��ͷ�ι� ���ٹ���ҩƷ�ܽ� AC
��������
��1������:��Ҫ�Ȼ��Ƶ�����Ϊ��60g��5%=3g����Ҫˮ������=60g-3g=57g����Ҫˮ�����Ϊ=![]() =57mL��
=57mL��
��2����ȡ�Ȼ���:�ڼ�����ƽ�����ƽ���˵������Ϸֱ����������ȵ�ֽƬ���ƶ��������̶����ͼ��ʾ����ʾΪ3g��
��3����ȡˮ:������Ͳ��ֱ�Ӽ�ˮ����ˮ�ӽ��̶�ʱ���ý�ͷ�ιܼ�����ˮ������̶ȡ�
��4���ܽ�:�ܽ�ʱ���ò����������Ŀ���ǣ����ٹ���ҩƷ�ܽ⣻
��5��ת��:�������Һ����ָ�����������ϱ�ǩ��
��6������ʵ���лᵼ��������Һ��������������ƫС���ǣ�
A���Ȼ��Ʒ�ĩ������������������ƫС��������������ƫС��
B������Ͳ��ȡˮʱ���Ӷ�������ʵ����ȡˮ�����ƫС��������������ƫ��
C��������Һ���ձ���������������ˮ��ϴ����ֻˮ������ƫ��������������ƫС��
D��ת�Ƽ���õ���Һʱ��������Һ�彦������Ӱ����������������
��ѡ��AC��