��Ŀ����
���پ��ж����¼�ʱ�з��������پơ�һ�����ɹ�ҵ�ƾ���ˮ���ƶ��ɣ�������һ�����ļ״�[CH3OH]�������ü״���ʹ������Ѹ���½���ʧ������������������������⣬��д���пհף�
��1����ҵ�ƾ�����Ҫ�ɷ����Ҵ�[C2H5OH]]���Ҵ���һ����Ҫ�Ļ���ԭ�ϣ���;�㷺�����д�2006������ȫ���ƹ�ʹ���Ҵ����ͣ��Ҵ��������������м���10%���Ҵ��γɵģ���д���Ҵ����ȼ�յĻ�ѧ����ʽ________��
��2����״����Ҵ��ṹ���ƵĻ����ﻹ�б���[C3H7OH]������[C4H9OH]���ȣ��������ʳ�Ϊ���࣮���ʣ�
���������еġ��ס��ҡ�����������������е�________�йأ�
�ں�n��̼ԭ�ӵĴ��Ļ�ѧʽΪ________��
�⣺��1���Ҵ��ij��ȼ�շ�Ӧ�����Ҵ��������������Ƕ�����̼��ˮ���ʴ�Ϊ��C2H5OH+3O2 2CO2+3H2O��
2CO2+3H2O��
��2���ټ״���ѧʽ��CH3OH���Ҵ���ѧʽ��C2H5OH��������ѧʽ��C3H7OH��������ѧʽ��C4H9OH������̼ԭ�Ӹ����������ӣ����úͼ��ұ���˳���Ǻϣ��ʴ�Ϊ��̼��ԭ�Ӹ�����
�ڹ۲�״���ѧʽCH3OH���Ҵ���ѧʽC2H5OH��������ѧʽC3H7OH��������ѧʽC4H9OH���ɿ�����ѧʽ�����ұ߲�����ͬ��ΪOH������ͬ��������ԭ�ӵĸ�����̼ԭ�Ӹ�����2����1���ʴ�Ϊ��CnH2n+1OH��
��������1�����ݻ�ѧ����ʽ��д�ķ���������д��
��2���ٴӸ����ʻ�ѧʽ�и�Ԫ��ԭ�ӵĸ������ɺ͡��ס��ҡ���������˳������յĽǶȽ��з�����
�ڷ������ִ��ѧʽ�и�Ԫ��ԭ�Ӹ�����������ϵ���Ӷ��ó���n��̼ԭ�ӵĴ��Ļ�ѧʽ��
������������Ҫ����ѧ����ѧ����ʽ��д�������Լ��۲�������������������л���ȼ�ջ�ѧ����ʽ����ƽ�����ù۲취��
 2CO2+3H2O��
2CO2+3H2O����2���ټ״���ѧʽ��CH3OH���Ҵ���ѧʽ��C2H5OH��������ѧʽ��C3H7OH��������ѧʽ��C4H9OH������̼ԭ�Ӹ����������ӣ����úͼ��ұ���˳���Ǻϣ��ʴ�Ϊ��̼��ԭ�Ӹ�����
�ڹ۲�״���ѧʽCH3OH���Ҵ���ѧʽC2H5OH��������ѧʽC3H7OH��������ѧʽC4H9OH���ɿ�����ѧʽ�����ұ߲�����ͬ��ΪOH������ͬ��������ԭ�ӵĸ�����̼ԭ�Ӹ�����2����1���ʴ�Ϊ��CnH2n+1OH��
��������1�����ݻ�ѧ����ʽ��д�ķ���������д��
��2���ٴӸ����ʻ�ѧʽ�и�Ԫ��ԭ�ӵĸ������ɺ͡��ס��ҡ���������˳������յĽǶȽ��з�����
�ڷ������ִ��ѧʽ�и�Ԫ��ԭ�Ӹ�����������ϵ���Ӷ��ó���n��̼ԭ�ӵĴ��Ļ�ѧʽ��
������������Ҫ����ѧ����ѧ����ʽ��д�������Լ��۲�������������������л���ȼ�ջ�ѧ����ʽ����ƽ�����ù۲취��

��ϰ��ϵ�д�
 ��ѧ�����ϵ�д�
��ѧ�����ϵ�д� �·Ƿ��̸����100��ϵ�д�
�·Ƿ��̸����100��ϵ�д�
�����Ŀ
 CH3OH����X�Ļ�ѧʽΪ______�����жϵ�������______��
CH3OH����X�Ļ�ѧʽΪ______�����жϵ�������______��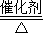 CH3OH����X�Ļ�ѧʽΪ______�����жϵ�������______��
CH3OH����X�Ļ�ѧʽΪ______�����жϵ�������______��