��Ŀ����
���پ��ж����¼�ʱ��������ȥ��5�£���ʡij�ؾ���پ��ж�����9�ˣ�
��1�����پơ�һ�����ɹ�ҵ�ƾ���ˮ���ƶ��ɵģ�������һ�����ļ״���CH3OH�������ü״���ʹ�˵�����Ѹ���½��������������״��Ĺ�ҵ�Ʒ�ΪX+2H2�TCH3OH����X�Ļ�ѧʽΪ
��2����ҵ�ƾ�����Ҫ�ɷ����Ҵ���C2H5OH�����Ҵ���һ����Ҫ�Ļ���ԭ�ϣ���;�㷺����д���Ҵ���ȼ��ȼ��ʱ����CO2��H2O�Ļ�ѧ����ʽ
��3����״����Ҵ��ṹ���ƵĻ�ѧ���ﻹ�б�����C3H7OH����������C4H9OH�����ȣ��������ʳ�Ϊ���࣮�� ���������еġ��ס��ҡ�����������������е�
��1�����پơ�һ�����ɹ�ҵ�ƾ���ˮ���ƶ��ɵģ�������һ�����ļ״���CH3OH�������ü״���ʹ�˵�����Ѹ���½��������������״��Ĺ�ҵ�Ʒ�ΪX+2H2�TCH3OH����X�Ļ�ѧʽΪ
CO
CO
�����жϵ������������غ㶨��
�����غ㶨��
����2����ҵ�ƾ�����Ҫ�ɷ����Ҵ���C2H5OH�����Ҵ���һ����Ҫ�Ļ���ԭ�ϣ���;�㷺����д���Ҵ���ȼ��ȼ��ʱ����CO2��H2O�Ļ�ѧ����ʽ
C2H5OH+3O2
2CO2+3H2O
| ||
C2H5OH+3O2
2CO2+3H2O
| ||
��3����״����Ҵ��ṹ���ƵĻ�ѧ���ﻹ�б�����C3H7OH����������C4H9OH�����ȣ��������ʳ�Ϊ���࣮�� ���������еġ��ס��ҡ�����������������е�
̼ԭ�Ӹ���
̼ԭ�Ӹ���
�йأ� �ں�n��̼ԭ�ӵĴ��Ļ�ѧʽΪCnH2n+1OH
CnH2n+1OH
����������1�����������غ㶨�ɣ���ѧ��Ӧǰ��Ԫ�ص�����䣬ԭ�ӵĸ������䣮
��2���Ҵ�ֻ��̼���⡢������Ԫ�أ�����Ҵ���ȫȼ�պ�ֻ���ɶ�����̼��ˮ��
��3���л�����������������̼ԭ�ӵĸ��������йأ�
��2���Ҵ�ֻ��̼���⡢������Ԫ�أ�����Ҵ���ȫȼ�պ�ֻ���ɶ�����̼��ˮ��
��3���л�����������������̼ԭ�ӵĸ��������йأ�
����⣺��1���ڻ�ѧ����ʽX+2H2
CH3OH�������1��X���Ӻ�4����ԭ�ӣ��ұ���1��̼ԭ�ӡ�4����ԭ�Ӻ�1����ԭ�ӣ����������غ㶨����Ԫ������䡢ԭ�Ӹ���������ص㣬��֪1��X�����к���1��̼ԭ�Ӻ�1����ԭ�ӣ����X�Ļ�ѧʽ��ʾΪCO��
��2���Ҵ��������ڵ�ȼ�����·�Ӧ���ɶ�����̼��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��C2H5OH+3O2
2CO2+3H2O��
��3���ɱ�����C3H7OH���Ͷ�����C4H9OH�������ƺͻ�ѧʽ���Կ������л���������������̼ԭ�ӵĸ�����Ӧ��������Ϊ����ij���������оͺ���3��̼ԭ�ӣ��Դ����ƣ������Կ����������ʵĻ�ѧʽ����ԭ�ӵĸ����ܱ�̼ԭ�Ӹ�����2����2�����������1����ԭ�ӣ���˴������ʵĻ�ѧʽ���Ա�ʾΪCnH2n+1OH��CnH2n+2O��
�ʴ�Ϊ��
��1��CO�������غ㶨�ɣ���2��C2H5OH+3O2
2CO2+3H2O����3��̼ԭ�Ӹ�����CnH2n+1OH��
| ||
| ���� |
��2���Ҵ��������ڵ�ȼ�����·�Ӧ���ɶ�����̼��ˮ���÷�Ӧ�Ļ�ѧ����ʽΪ��C2H5OH+3O2
| ||
��3���ɱ�����C3H7OH���Ͷ�����C4H9OH�������ƺͻ�ѧʽ���Կ������л���������������̼ԭ�ӵĸ�����Ӧ��������Ϊ����ij���������оͺ���3��̼ԭ�ӣ��Դ����ƣ������Կ����������ʵĻ�ѧʽ����ԭ�ӵĸ����ܱ�̼ԭ�Ӹ�����2����2�����������1����ԭ�ӣ���˴������ʵĻ�ѧʽ���Ա�ʾΪCnH2n+1OH��CnH2n+2O��
�ʴ�Ϊ��
��1��CO�������غ㶨�ɣ���2��C2H5OH+3O2
| ||
������������Ҫ���黯ѧʽ���ƶϺͻ�ѧ����ʽ����д���������ʵ������ͻ�ѧʽ�Ĺ��ɣ��Ѷ��Դ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 CH3OH����X�Ļ�ѧʽΪ______�����жϵ�������______��
CH3OH����X�Ļ�ѧʽΪ______�����жϵ�������______��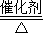 CH3OH����X�Ļ�ѧʽΪ______�����жϵ�������______��
CH3OH����X�Ļ�ѧʽΪ______�����жϵ�������______��