ĢāÄæÄŚČŻ
£Ø2013?Ģ«Ō£©»ÆѧŠ”×éĶ¬Ń§ĪŖ¼ų¶Øij¹¤³§ÅųöµÄĪŽÉ«ĪŪĖ®³É·Ö£¬ĖūĆĒ²éŌÄøĆ¹¤³§µÄÓŠ¹Ų׏ĮĻŗó£¬ĶĘ²ā³öĪŪĖ®ÖŠæÉÄÜŗ¬ÓŠHCl”¢Na2SO4”¢Na2CO3ÖŠµÄŅ»ÖÖ»ņ¼øÖÖĪļÖŹ£¬Ķ¬Ń§ĆĒČ”ĪŪĖ®½ųŠŠĮĖČēĻĀĢ½¾æ£ŗ£ØŅŃÖŖNa2SO4ČÜŅŗ³ŹÖŠŠŌ£©
£Ø1£©²āĪŪĖ®µÄpH£ŗČ”Ņ»ÕÅpHŹŌÖ½£¬·ÅŌŚ²£Į§Ę¬ÉĻ
£Ø2£©¼ģŃéNa2SO4ŹĒ·ń“ęŌŚ£ŗ¼×Ķ¬Ń§ŌŚŅ»Ö§ŹŌ¹Ü֊ȔɣĮæĪŪĖ®£¬ĻņŹŌ¹ÜÖŠµĪ¼ÓÉŁĮæµÄ
ĶعżĢ½¾æ£¬Ķ¬Ń§ĆĒČ·¶ØĮĖĪŪĖ®µÄ³É·Ö£®
ĪŖŹ¹ÅŷŵÄĪŪĖ®ÖŠ²»ŗ¬ÓŠĖį»ņ¼ī£¬æÉĻņĪŪĖ®ÖŠ¼Ó¹żĮæµÄ
£Ø1£©²āĪŪĖ®µÄpH£ŗČ”Ņ»ÕÅpHŹŌÖ½£¬·ÅŌŚ²£Į§Ę¬ÉĻ
ÓĆ²£Į§°ōÕŗČ”ĪŪĖ®ŃłĘ·£¬µĪŌŚpHŹŌÖ½ÉĻ£¬Óė±ź×¼±ČÉ«æØ±Č½Ļ
ÓĆ²£Į§°ōÕŗČ”ĪŪĖ®ŃłĘ·£¬µĪŌŚpHŹŌÖ½ÉĻ£¬Óė±ź×¼±ČÉ«æØ±Č½Ļ
£¬¶ĮŹżĪŖPh=2£¬ÓÉ“ĖæÉÖŖ£ŗĪŪĖ®ÖŠŅ»¶ØÓŠHCl
HCl
£¬æÉÄÜÓŠNa2SO4£®£Ø2£©¼ģŃéNa2SO4ŹĒ·ń“ęŌŚ£ŗ¼×Ķ¬Ń§ŌŚŅ»Ö§ŹŌ¹Ü֊ȔɣĮæĪŪĖ®£¬ĻņŹŌ¹ÜÖŠµĪ¼ÓÉŁĮæµÄ
BaCl2
BaCl2
ČÜŅŗ£¬Õńµ“£¬²śÉśĮĖ°×É«³Įµķ£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖNa2SO4+BaCl2ØTBaSO4”ż+2NaCl
Na2SO4+BaCl2ØTBaSO4”ż+2NaCl
£ØŗĻĄķ¼“æÉ£©£¬Ö¤Ć÷ĪŪĖ®ÖŠÓŠNa2SO4“ęŌŚ£®ŅŅĶ¬Ń§ÓĆĪļĄķ·½·ØŅ²µĆµ½ĮĖĶ¬ŃłµÄ½įĀŪ£¬ĖūµÄŹµŃé²Ł×÷¼°ĻÖĻóŹĒŌŚŹŌ¹Ü֊ȔɣĮæĪŪĖ®ŃłĘ·ÕōøÉ£¬ÓŠ°×É«¹ĢĢåĪö³ö
ŌŚŹŌ¹Ü֊ȔɣĮæĪŪĖ®ŃłĘ·ÕōøÉ£¬ÓŠ°×É«¹ĢĢåĪö³ö
£®ĶعżĢ½¾æ£¬Ķ¬Ń§ĆĒČ·¶ØĮĖĪŪĖ®µÄ³É·Ö£®
ĪŖŹ¹ÅŷŵÄĪŪĖ®ÖŠ²»ŗ¬ÓŠĖį»ņ¼ī£¬æÉĻņĪŪĖ®ÖŠ¼Ó¹żĮæµÄ
ŹÆ»ŅŹÆ£Ø»ņĢśŠ¼£©
ŹÆ»ŅŹÆ£Ø»ņĢśŠ¼£©
£ØŗĻĄķ¼“æÉ£©£®·ÖĪö£ŗ£Ø1£©øł¾ŻČÜŅŗpHÖµµÄ²ā¶Ø·½·ØŅŌ¼°ŃĪĖįĻŌĖįŠŌpHŠ”ÓŚ7½ųŠŠ½ā“š£»
£Ø2£©øł¾ŻĮņĖįÄĘČÜŅŗŗĶĀČ»Æ±µČÜŅŗ·“Ӧɜ³ÉĮņĖį±µ°×É«³Įµķ”¢ŃĪĖįŅ×»Ó·¢ŅŌ¼°ŃĪĖįŗĶŹÆ»ŅŹÆ»ņĢśŠ¼·“Ó¦½ųŠŠ½ā“š£®
£Ø2£©øł¾ŻĮņĖįÄĘČÜŅŗŗĶĀČ»Æ±µČÜŅŗ·“Ӧɜ³ÉĮņĖį±µ°×É«³Įµķ”¢ŃĪĖįŅ×»Ó·¢ŅŌ¼°ŃĪĖįŗĶŹÆ»ŅŹÆ»ņĢśŠ¼·“Ó¦½ųŠŠ½ā“š£®
½ā“š£ŗ½ā£ŗ£Ø1£©²āĪŪĖ®µÄpH£ŗČ”Ņ»ÕÅpHŹŌÖ½£¬·ÅŌŚ²£Į§Ę¬ÉĻÓĆ²£Į§°ōÕŗČ”ĪŪĖ®ŃłĘ·£¬µĪŌŚpHŹŌÖ½ÉĻ£¬Óė±ź×¼±ČÉ«æØ±Č½Ļ£¬¶ĮŹżĪŖpH=2£¬ÓÉ“ĖæÉÖŖ£ŗĪŪĖ®ÖŠŅ»¶ØÓŠŃĪĖį£¬Ņ»¶ØƻӊĢ¼ĖįÄĘ£¬æÉÄÜÓŠNa2SO4£»
£Ø2£©ĮņĖįÄĘČÜŅŗŗĶĀČ»Æ±µČÜŅŗ·“Ӧɜ³ÉĮņĖį±µ°×É«³Įµķ£¬ĖłŅŌ¼ģŃéNa2SO4ŹĒ·ń“ęŌŚ£ŗ¼×Ķ¬Ń§ŌŚŅ»Ö§ŹŌ¹Ü֊ȔɣĮæĪŪĖ®£¬ĻņŹŌ¹ÜÖŠµĪ¼ÓÉŁĮæµÄĀČ»Æ±µČÜŅŗ£¬Õńµ“£¬²śÉśĮĖ°×É«³Įµķ£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖNa2SO4+BaCl2=BaSO4”ż+2NaCl£»ŃĪĖįŅ×»Ó·¢£¬ĖłŅŌÖ¤Ć÷ĪŪĖ®ÖŠÓŠNa2SO4“ęŌŚ£®ŅŅĶ¬Ń§ÓĆĪļĄķ·½·ØŅ²µĆµ½ĮĖĶ¬ŃłµÄ½įĀŪ£¬ĖūµÄŹµŃé²Ł×÷¼°ĻÖĻóŹĒ£ŗŌŚŹŌ¹Ü֊ȔɣĮæĪŪĖ®ŃłĘ·ÕōøÉ£¬ÓŠ°×É«¹ĢĢåĪö³ö£®ŃĪĖįŗĶŹÆ»ŅŹÆ»ņĢśŠ¼·“Ó¦£¬ĖłŅŌĪŖŹ¹ÅŷŵÄĪŪĖ®ÖŠ²»ŗ¬ÓŠĖį»ņ¼ī£¬æÉĻņĪŪĖ®ÖŠ¼Ó¹żĮæµÄŹÆ»ŅŹÆ£Ø»ņĢśŠ¼£©£®
¹Ź“š°øĪŖ£ŗ£Ø1£©ÓĆ²£Į§°ōÕŗČ”ĪŪĖ®ŃłĘ·£¬µĪŌŚpHŹŌÖ½ÉĻ£¬Óė±ź×¼±ČÉ«æØ±Č½Ļ£»HCl£»
£Ø2£©BaCl2£»Na2SO4+BaCl2=BaSO4”ż+2NaCl£»ŌŚŹŌ¹Ü֊ȔɣĮæĪŪĖ®ŃłĘ·ÕōøÉ£¬ÓŠ°×É«¹ĢĢåĪö³ö£»ŹÆ»ŅŹÆ£Ø»ņĢśŠ¼£©£®
£Ø2£©ĮņĖįÄĘČÜŅŗŗĶĀČ»Æ±µČÜŅŗ·“Ӧɜ³ÉĮņĖį±µ°×É«³Įµķ£¬ĖłŅŌ¼ģŃéNa2SO4ŹĒ·ń“ęŌŚ£ŗ¼×Ķ¬Ń§ŌŚŅ»Ö§ŹŌ¹Ü֊ȔɣĮæĪŪĖ®£¬ĻņŹŌ¹ÜÖŠµĪ¼ÓÉŁĮæµÄĀČ»Æ±µČÜŅŗ£¬Õńµ“£¬²śÉśĮĖ°×É«³Įµķ£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖNa2SO4+BaCl2=BaSO4”ż+2NaCl£»ŃĪĖįŅ×»Ó·¢£¬ĖłŅŌÖ¤Ć÷ĪŪĖ®ÖŠÓŠNa2SO4“ęŌŚ£®ŅŅĶ¬Ń§ÓĆĪļĄķ·½·ØŅ²µĆµ½ĮĖĶ¬ŃłµÄ½įĀŪ£¬ĖūµÄŹµŃé²Ł×÷¼°ĻÖĻóŹĒ£ŗŌŚŹŌ¹Ü֊ȔɣĮæĪŪĖ®ŃłĘ·ÕōøÉ£¬ÓŠ°×É«¹ĢĢåĪö³ö£®ŃĪĖįŗĶŹÆ»ŅŹÆ»ņĢśŠ¼·“Ó¦£¬ĖłŅŌĪŖŹ¹ÅŷŵÄĪŪĖ®ÖŠ²»ŗ¬ÓŠĖį»ņ¼ī£¬æÉĻņĪŪĖ®ÖŠ¼Ó¹żĮæµÄŹÆ»ŅŹÆ£Ø»ņĢśŠ¼£©£®
¹Ź“š°øĪŖ£ŗ£Ø1£©ÓĆ²£Į§°ōÕŗČ”ĪŪĖ®ŃłĘ·£¬µĪŌŚpHŹŌÖ½ÉĻ£¬Óė±ź×¼±ČÉ«æØ±Č½Ļ£»HCl£»
£Ø2£©BaCl2£»Na2SO4+BaCl2=BaSO4”ż+2NaCl£»ŌŚŹŌ¹Ü֊ȔɣĮæĪŪĖ®ŃłĘ·ÕōøÉ£¬ÓŠ°×É«¹ĢĢåĪö³ö£»ŹÆ»ŅŹÆ£Ø»ņĢśŠ¼£©£®
µćĘĄ£ŗ±¾Ģāæ¼²éĮĖ³£¼ū»ģŗĻĪļ×é³ÉµÄĶʶĻ£¬Ķź³É“ĖĄąĢāÄ棬¹Ų¼üŹĒøł¾ŻĢāøÉŠšŹöµÄŹµŃéĻÖĻó£¬ŅĄ¾ŻĪļÖŹµÄŠŌÖŹ×÷³öŗĻĄķµÄĶĘ²ā£®

Į·Ļ°²įĻµĮŠ“š°ø
Ļą¹ŲĢāÄæ

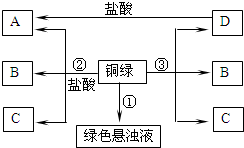 £Ø2013?ČēøŽŹŠÄ£Äā£©Š”Ķ®µÄŅ»ø±ŃŪ¾µ“÷ĮĖŅ»¶ĪŹ±¼äŗ󣬷¢ĻÖĶÖŹ¾µ¼ÜÉĻ³öĻÖĮĖŅ»²ćĀĢÉ«ĪļÖŹ£¬ĖūĻė½«Ęä³żµō£®¾²éŌÄ׏ĮĻµĆÖŖ£ŗĶŌŚŅ»¶ØĢõ¼žĻĀ»įŠāŹ“Éś³ÉŅ»ÖÖĀĢÉ«ĪļÖŹ£¬ĘäÖ÷ŅŖ³É·ÖŹĒ¼īŹ½Ģ¼ĖįĶ[Ė׳ĘĶĀĢ£¬»ÆѧŹ½ĪŖCu2£ØOH£©2CO3]£¬øĆĪļÖŹŹÜČČ²»ĪȶØŅ×·Ö½ā£®Ėūøł¾ŻŌŖĖŲŹŲŗć·ÖĪö£¬µĆ³öĶÉśŠāŹĒĶÓėæÕĘųÖŠµÄŃõĘų”¢¶žŃõ»ÆĢ¼ŗĶ
£Ø2013?ČēøŽŹŠÄ£Äā£©Š”Ķ®µÄŅ»ø±ŃŪ¾µ“÷ĮĖŅ»¶ĪŹ±¼äŗ󣬷¢ĻÖĶÖŹ¾µ¼ÜÉĻ³öĻÖĮĖŅ»²ćĀĢÉ«ĪļÖŹ£¬ĖūĻė½«Ęä³żµō£®¾²éŌÄ׏ĮĻµĆÖŖ£ŗĶŌŚŅ»¶ØĢõ¼žĻĀ»įŠāŹ“Éś³ÉŅ»ÖÖĀĢÉ«ĪļÖŹ£¬ĘäÖ÷ŅŖ³É·ÖŹĒ¼īŹ½Ģ¼ĖįĶ[Ė׳ĘĶĀĢ£¬»ÆѧŹ½ĪŖCu2£ØOH£©2CO3]£¬øĆĪļÖŹŹÜČČ²»ĪȶØŅ×·Ö½ā£®Ėūøł¾ŻŌŖĖŲŹŲŗć·ÖĪö£¬µĆ³öĶÉśŠāŹĒĶÓėæÕĘųÖŠµÄŃõĘų”¢¶žŃõ»ÆĢ¼ŗĶ