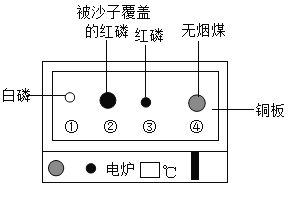
��Ŀ����
����Ŀ�����ñ���װ�ý�������ʵ�顣
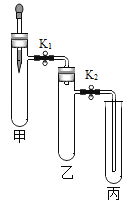
ʵ��װ�� | ʵ��1 | ʵ��2 |
| �ټ��Թ��з�������MnO2���ι��з������������Һ���ҡ����и�����һС����ף����μ���������80����ˮ��������ȼ�� �ڴ�K1��K2��������������Һ�����Թ��У��۲쵽���а��ײ�ȼ�գ����а���ȼ�� ��һ��ʱ����е�����Һ������ | �ٹر�K1����K2�����Թ��г���CO2���ι��з���ŨNaOH��Һ�����Թ��з������������ۣ����з���Լ���Թܵ�ϡ���� �ڽ�ŨNaOH��Һ������Թ��У�һ��ʱ���K1 |
��1��ʵ��1�����з�����Ӧ�Ļ�ѧ����ʽΪ_____���Ա��Һͱ��е�ʵ������˵����ʵ����̽����ȼ��������_____��
��2��ʵ��1������۵�����˵��װ����ѹǿ��С��ѹǿ��С��ԭ����_____��
��3��ʵ��2�����з�����Ӧ�Ļ�ѧ����ʽΪ_____����K1�����е�������_____��
���𰸡�2H2O2![]() 2H2O + O2�� �������Ӵ� װ�ü��з�Ӧ���ȣ�һ��ʱ����¶Ƚ��ͣ�����ѹǿ��С CO2 + 2NaOH=Na2CO3+ H2O ���е�ϡ���ᵹ�������У�����ɫ�����ݲ�������Һ����ɫ��Ϊdz��ɫ
2H2O + O2�� �������Ӵ� װ�ü��з�Ӧ���ȣ�һ��ʱ����¶Ƚ��ͣ�����ѹǿ��С CO2 + 2NaOH=Na2CO3+ H2O ���е�ϡ���ᵹ�������У�����ɫ�����ݲ�������Һ����ɫ��Ϊdz��ɫ
��������
��1��ʵ��1�����з�����Ӧ����������ȡ��������Ӧ�ķ���ʽΪ2H2O2![]() 2H2O + O2�� ���ҡ����и�����һС����ף����μ���������80����ˮ����K1��K2��������������Һ�����Թ��У��۲쵽���а��ײ�ȼ�գ����а���ȼ�գ��ұ��IJ�֮ͬ���ǵ��ܵij��ȣ����еĵ��ܿ��Խ�����ͨ�뵽�Թܵײ�����Ӵ����Ҳ����ԣ����Ա�ʵ�����̽��ȼ����Ҫ�����μӵ����������2H2O2
2H2O + O2�� ���ҡ����и�����һС����ף����μ���������80����ˮ����K1��K2��������������Һ�����Թ��У��۲쵽���а��ײ�ȼ�գ����а���ȼ�գ��ұ��IJ�֮ͬ���ǵ��ܵij��ȣ����еĵ��ܿ��Խ�����ͨ�뵽�Թܵײ�����Ӵ����Ҳ����ԣ����Ա�ʵ�����̽��ȼ����Ҫ�����μӵ����������2H2O2![]() 2H2O + O2�� ���������Ӵ���
2H2O + O2�� ���������Ӵ���
��2��ʵ��1������۵�����һ��ʱ����е�����Һ��������˵��װ����ѹǿ��С��ѹǿ��С��ԭ����װ�ü��з�Ӧ���ȣ�һ��ʱ�������ɢʧ���¶Ƚ��ͣ�����ѹǿ��С�����װ�ü��з�Ӧ���ȣ�һ��ʱ����¶Ƚ��ͣ�����ѹǿ��С��
��3��ʵ��2�����н�ͷ�ι�������������Һ�����Թ���ʢ�Ŷ�����̼����ŨNaOH��Һ������Թ��У����߷�Ӧ����̼���ƺ�ˮ��������Ӧ�Ļ�ѧ����ʽΪCO2 + 2NaOH=Na2CO3+ H2O����K1�����ڶ�����̼���������Ʒ�Ӧ�������Թ��е�ѹǿС��������ѹ�����Թ��е�ϡ����ᱻѹ�����Թ��У����������������Ӧ�����Ȼ��������������ʻῴ�����Թ��в����������ݣ���Һ����ɫ��Ϊdz��ɫ��������е�ϡ���ᵹ�������У�����ɫ�����ݲ�������Һ����ɫ��Ϊdz��ɫ��

����Ŀ��������ˮ����500 mL������ƿ�м���2�װ��ǡ�1.5 gС�մ�ע������ˮ���ټ���1.5 g�����ᣨC6H8O7������������ƿ�ǣ�ҡ�ȡ�
�����ϣ���Ӧԭ����3NaHCO3 + C6H8O7=C6H5O7Na3 + 3H2O+ 3CO2��
���A��B��������ѡ1������������������𣬰�A�Ʒ֡�
A | B |
��1����������̼����Ԫ�ص�������Ϊ_____�� ��2���Ƶõ���ˮ�������ζ���������ݲ�������ζ��������_____�� | ��1����������̼Ԫ�ص���������Ϊ37.5%�������ʽΪ_____�� ��2���������������������ƿ�ǵ�ԭ����_____�� |
����Ŀ����ʷ�ϵ�˿��֮·��Ҫ����Ʒ��ͨ���ޣ������һ��һ·ͬ���Ǻ����빲Ӯ��
| ��� | ���� |
�侮������ұ������������˿����������Ҷ�� | ��Ѫ��������Ƥ�������ѡ��ƹϡ����ܲ����϶����鱦���Ρ�ҩ�ġ����ϵ� | |
| ���ߡ���·���ۿڡ����������š��˵�Ȼ����豸����Դ�豸���ճ���Ʒ�� | ��Ʒ�ͺ�ʯ������Ʒ����Ȼ���� |
��1���Ŵ�ұ������ʪ����ͭ�ķ�Ӧԭ����_____���ִ���·��������Ҫ�����ĸ�������ҵ�ú�80���������ij������������������ܵõ���_____�֡�
��2����Ȼ����Ҫ�ɷ��Ǽ��飬�ڼ�����̼Ԫ������Ԫ�ص���������_____��