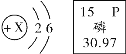��Ŀ����
����Ŀ����1��������ѧ��ѧ֪ʶ�����������⣺
���������γ���������Ĥ�Ļ�ѧʽ_________________________��
�� ![]() �С�+2����ʾ________
�С�+2����ʾ________
��2����������(NaN3)���㷺Ӧ����������ȫ���ҡ��������Ƶ��Ʊ�������������Һ̬����Ӧ��NaNH2���ٽ�NaNH2��N2O��Ӧ������NaN3���÷�Ӧ�Ļ�ѧ����ʽΪ��
2NaNH2 + N2O=NaN3 + NaOH + X����ش��������⣺
��N2O��������________��X�Ļ�ѧʽΪ_______________��
��NaNH2�е�Ԫ�صĻ��ϼ�Ϊ____________������H��N��NaԪ�ص�������Ϊ____________
��NaN3�е�������Ϊ_________��
��3��Ϊ��������β���ŷţ��ҹ�����ȫ����Χ���ƹ㳵���Ҵ����͡�

�����Ͳ���ȫȼ�ջ����CO��̼�⻯����Ҵ���C2H5OH����ȼ�ձ�������֣��Ҵ���ȫȼ�յĻ�ѧ����ʽΪ__________��
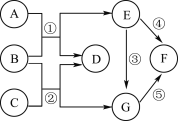
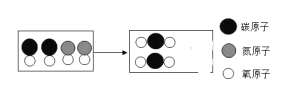
������β���к���CO��NO����Ⱦ�ʹ����Ԫ�������Խ�����ת����CO2��N2������ʾ��ͼ����ͼ��ʾ�����ڷ����в�ȫ��Ӧ����ͼʾ��_____
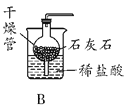
��4��ijͬѧ����ͼ��ʾװ���о������غ㶨�ɵ�������⡣��Ӧǰ�Ƶ�������Ϊm1��������ϡ����ȫ�������ձ�����ַ�Ӧ�Ƶ�������Ϊm2��
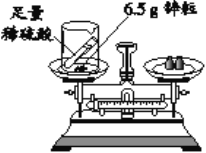
��m1��m2��ԭ����_________ ���û�ѧ����ʽ��ʾ�����÷�Ӧ�Ƿ����������غ㶨��_________����ǡ�����
��������m1��m2�IJ�ֵ��_________ g��
���𰸡�Al2O3 �Ȼ������У���Ԫ��Ϊ������ һ�������� NH3 ��3�� 2:14:23 Na+ C2H5OH + 3O2 ![]() 2CO2 + 3H2O
2CO2 + 3H2O ![]() Zn + H2SO4 === ZnSO4 + H2�� �� 0.2
Zn + H2SO4 === ZnSO4 + H2�� �� 0.2
��������
��1�����������γɵ���������Ĥ�����������仯ѧʽΪAl2O3������Al2O3��
�ڡ�+2����ʾ�Ȼ������У���Ԫ�صĻ��ϼ�Ϊ�������������Ȼ������У���Ԫ�صĻ��ϼ�Ϊ��������
��2����N2O��������һ��������������һ����������
�ɻ�ѧ����ʽ��֪����ѧ����ʽ��ǰ�沿�ֳ�����2����ԭ�ӣ�4����ԭ�ӣ�4����ԭ�Ӻ�1����ԭ�ӣ���ѧ����ʽ�ĺ��沿���ѳ���2����ԭ�ӣ�3����ԭ�ӣ�1����ԭ�Ӻ�1����ԭ�ӣ���ȱ��1����ԭ�Ӻ�3����ԭ�Ӿʹ�����X������X�Ļ�ѧʽΪNH3������NH3����NaNH2�У���Ԫ�صĻ��ϼ�Ϊ+1�ۣ���Ԫ�صĻ��ϼ�Ϊ+1�ۣ��������и�Ԫ�صĻ��ϼ۵Ĵ�����Ϊ�㣬�赪Ԫ�صĻ��ϼ�Ϊx����+1+x+��+1����2=0��x=3������3��
NaNH2����H��N��NaԪ�ص�������Ϊ��1��2��:14:23=2:14:23������2:14:23��
��NaN3�е��������������ӣ������ΪNa+������Na+��
��3�����Ҵ��������ڵ�ȼ�������·�Ӧ���ɶ�����̼��ˮ���ʷ�Ӧ�Ļ�ѧ����ʽдΪ��. C2H5OH + 3O2 ![]() 2CO2 + 3H2O��
2CO2 + 3H2O��
����ͼ��֪��ͼ��ȱ�ٵ����ǵ����ӣ�����![]() ��
��
��4����ʵ����п�����ᷴӦ��������п���������ʷ�Ӧ�Ļ�ѧ����ʽдΪ��Zn + H2SO4=ZnSO4 + H2����
��ѧ��Ӧ����ѭ�����غ㶨�ɣ��÷�ӦҲ���ڻ�ѧ�仯��Ҳ����ѭ�����غ㶨�ɵģ���������
����ʵ��ǰ�����������Ƿ�Ӧ�����ɵ�������������6.5gп������ϡ���ᷴӦ��������������Ϊ0.2g������0.2��