��Ŀ����
�����ͼʾ�ش��������⣺


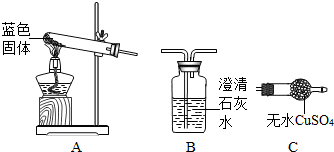
��1��д��ͼһ�б�ŵ��������ƣ���__________���� ______________��
��2��ijͬѧ����ͼһװ����ȡ��������ѡ��������ǣ��ѧʽ��__________���ø�������ȡ�����Ļ�ѧ����ʽΪ ____________��
��3��ij��ѧС��ͬѧ�����������ȡ��������֤�����װ��(��ͼ��)����Һ©�������ų�һ��������������Һ���ϻ�����A�г��ִ������ݣ�B�а���ȼ�գ�C��Һ���½���ϡ��������D�С��뿴ͼ�ش��������⣺
��B�а����ܹ���ˮ��ȼ�յ�ԭ����__________��
��E�е�ʵ��������_________��
����Fװ���ռ������������_____________��
�� D�з�Ӧ�Ļ�ѧ����ʽΪ__________��
��2��ijͬѧ����ͼһװ����ȡ��������ѡ��������ǣ��ѧʽ��__________���ø�������ȡ�����Ļ�ѧ����ʽΪ ____________��
��3��ij��ѧС��ͬѧ�����������ȡ��������֤�����װ��(��ͼ��)����Һ©�������ų�һ��������������Һ���ϻ�����A�г��ִ������ݣ�B�а���ȼ�գ�C��Һ���½���ϡ��������D�С��뿴ͼ�ش��������⣺
��B�а����ܹ���ˮ��ȼ�յ�ԭ����__________��
��E�е�ʵ��������_________��
����Fװ���ռ������������_____________��
�� D�з�Ӧ�Ļ�ѧ����ʽΪ__________��
��1�� ���Թ� �� ������̨
��2��KMnO4 ��KClO3��MnO2 ��2KMnO4 K2MnO4+MnO2+O2����2KClO3
K2MnO4+MnO2+O2����2KClO3 2KCl + 3O2��
2KCl + 3O2��
��3�����ṩ������ �� ����Һ��� �������ܶȴ��ڿ������ܶ� ���� CaCO3 + 2HCl == CaCl2 + H2O + CO2��
��2��KMnO4 ��KClO3��MnO2 ��2KMnO4
 K2MnO4+MnO2+O2����2KClO3
K2MnO4+MnO2+O2����2KClO3 2KCl + 3O2��
2KCl + 3O2����3�����ṩ������ �� ����Һ��� �������ܶȴ��ڿ������ܶ� ���� CaCO3 + 2HCl == CaCl2 + H2O + CO2��

��ϰ��ϵ�д�
 ��ս�п�����ϵ�д�
��ս�п�����ϵ�д�
�����Ŀ