��Ŀ����
21������������ũҵ�������벻��ˮ����ͼ������ˮ����ˮ����ʾ��ͼ��
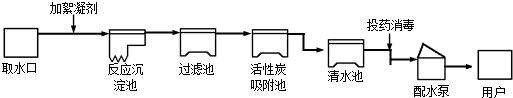
�����ͼʾ�ش��������⣺
��1����ͼ�������ڵĻ���̿��
��2����ͥ�����п�����
��3�����õ���Դˮ�к��н϶��MgCl2����д����Դˮ���������м���CaOʱ�йػ�ѧ����ʽ��
��

�����ͼʾ�ش��������⣺
��1����ͼ�������ڵĻ���̿��
����
���ã������û�л���̿��������������ľ̿
����ˮ����2����ͥ�����п�����
����ˮ
����ijˮ����Ӳˮ������ˮ����3�����õ���Դˮ�к��н϶��MgCl2����д����Դˮ���������м���CaOʱ�йػ�ѧ����ʽ��
CaO+H2O�TCa��OH��2
����
Ca��OH��2+MgCl2=CaCl2+Mg��OH��2��
������������������ˮ������ͼʾ���ۺϿ���ѧ���Ծ�ˮ������Ӧ�ã�Ҫ��ѧ�����⾻ˮ�ĸ�������ͬ��ͬʱ���漰Ӳˮ��������ѧ����ʽ����д��
����⣺��1������̿���к�ǿ������������������������ڵĻ���̿��Ҫ�����������ã���ũ��û�л���̿��Ҳ������ľ̿���������棬����Ҳ���������ԣ�
��2������Ӳˮ����ˮ������ķ������÷���ˮ���д�����������Ӳˮ��û�л���ٵ�����ˮ��
��3����ʯ���������ƣ���ˮ��Ӧ�����������ƣ�����������ˮ�е��Ȼ�þ��Ӧ�����Ȼ��ƺ�������þ����ѧ����ʽΪCaO+H2O�TCa��OH��2��Ca��OH��2+MgCl2=CaCl2+Mg��OH��2����
�ʴ�Ϊ����1��������������ľ̿��
��2�� ����ˮ��
��3��CaO+H2O�TCa��OH��2��Ca��OH��2+MgCl2=CaCl2+Mg��OH��2����
��2������Ӳˮ����ˮ������ķ������÷���ˮ���д�����������Ӳˮ��û�л���ٵ�����ˮ��
��3����ʯ���������ƣ���ˮ��Ӧ�����������ƣ�����������ˮ�е��Ȼ�þ��Ӧ�����Ȼ��ƺ�������þ����ѧ����ʽΪCaO+H2O�TCa��OH��2��Ca��OH��2+MgCl2=CaCl2+Mg��OH��2����
�ʴ�Ϊ����1��������������ľ̿��
��2�� ����ˮ��
��3��CaO+H2O�TCa��OH��2��Ca��OH��2+MgCl2=CaCl2+Mg��OH��2����
�����������Ϊ������д��ѧ����ʽ����һ���Ѷȣ�Ҫע����ƽ�ͳ������ţ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ