��Ŀ����

��1����ͼ�У�ʵ��A��̽��������̼���ܽ���ʵ�飮������̼�Ƿ��������ˮ������ֱ�ӹ۲쵽������ͨ��
��2����ͼ�У�ʵ��B��̽����ȼ������������ͨ�������Ƿ��������Ӵ��Ϳ���
��3����ͼ�У�ʵ��C��̽������������������м��Թܵ�ʵ��Ŀ����
�ۺϷ�������C��D����ʵ�飬���Ƕ�������
���㣺������̼����������,������ʴ�������������,��д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ,ȼ����ȼ�յ�����
ר�⣺ʵ���Լ����
��������1�����������̼��������ˮ����ƿ�ڵ�����ѹǿ��С������ƿ�ӱ��
��2��ȼ�յ���������ȼ��Ƿ������Ӵ����¶ȣ����¶��Ƿ�ﵽ�Ż�㣩��
��3���������������ˮ�֣�������C��D����ʵ�飬���Ƕ������˶Ա�ʵ�飻
��2��ȼ�յ���������ȼ��Ƿ������Ӵ����¶ȣ����¶��Ƿ�ﵽ�Ż�㣩��
��3���������������ˮ�֣�������C��D����ʵ�飬���Ƕ������˶Ա�ʵ�飻
����⣺��1��ʵ��A��̽��������̼���ܽ���ʵ�飬ͨ��ѹǿ�仯����֪��
��2��ʵ��C��̽����ȼ�����������öԱ�ʵ�飻
��3��ʵ��D��̽�����������������Ҳ�����öԱ�ʵ�飻
�ʴ�Ϊ����1��ƿ�ӱ��
��2���¶ȣ����¶��Ƿ�ﵽ�Ż�㣩��4P+5O2
2P2O5
��3����֤����ֻ��ˮ���������ܷ����⣻�Ա�
��2��ʵ��C��̽����ȼ�����������öԱ�ʵ�飻
��3��ʵ��D��̽�����������������Ҳ�����öԱ�ʵ�飻
�ʴ�Ϊ����1��ƿ�ӱ��
��2���¶ȣ����¶��Ƿ�ﵽ�Ż�㣩��4P+5O2
| ||
��3����֤����ֻ��ˮ���������ܷ����⣻�Ա�
���������⿼�������̽�����ʵ����ʺͱ仯���ɣ��˽������ܽ�ʱ�����Ⱥͷ�������ѧ��̽��ȼ�յ��������������������ʵ�鷽����

��ϰ��ϵ�д�
�����Ŀ
�������ʳ��ڷ���һ��ʱ���������ѧ�仯���ʣ��������������ǣ�������
| A��Ũ���� | B��Ũ���� |
| C��ʳ�Σ��Ȼ��ƣ� | D���ռ� |
����ʵ�������ȷ���ǣ�������
| A��ϡ��Ũ����ʱ����ˮ����Ũ���� |
| B������ƽ���������̷����룬���̷�ҩƷ |
| C����ȼ��ȼ������ǰ��һ��Ҫ�鴿 |
| D��CO��ԭCuO����ʱ����ͣ������Ϩ�� |
���б仯�����ڻ�ѧ�仯���ǣ�������
| A���û���̿����ˮ |
| B������ʯ���� |
| C��ֲ��Ĺ������ |
| D��ú��ȼ�� |


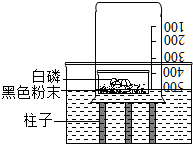 �ڰ��鱾װ�òⶨ����������������ʵ��ʱ����װ���ڵ���������һ������ʱ�����Ͳ�����������Ӧ���Ӷ����²������ƫС������ʦ�Ը�ʵ������˸Ľ���װ����ͼ��ʾ����һ�����е���ղ��ġ�ů��������ɫ��ĩ����������������İ��ף�Ѹ�ٿ����ձ�����������ȼ��������һװ�ÿɸ�ȷ�زⶨ���������������������
�ڰ��鱾װ�òⶨ����������������ʵ��ʱ����װ���ڵ���������һ������ʱ�����Ͳ�����������Ӧ���Ӷ����²������ƫС������ʦ�Ը�ʵ������˸Ľ���װ����ͼ��ʾ����һ�����е���ղ��ġ�ů��������ɫ��ĩ����������������İ��ף�Ѹ�ٿ����ձ�����������ȼ��������һװ�ÿɸ�ȷ�زⶨ���������������������