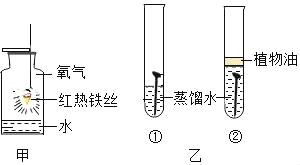
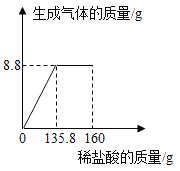
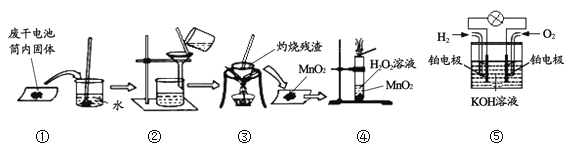
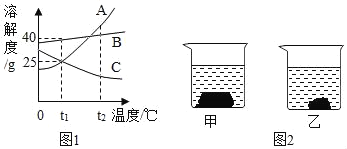
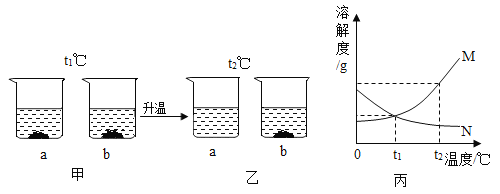
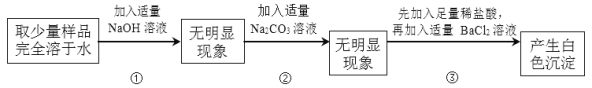
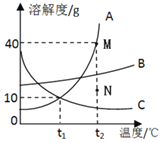
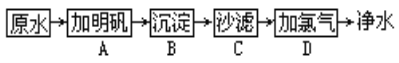
��Ŀ����
����Ŀ���ԷϾ��ֻ��������ÿɽ�Լ������Դ��ij�ֻ���·���к������½�����Ni��������Pb��Ǧ����Ag��Au��Fe��Cu��Sn��������Pd���٣�����ͼ��ij������ƵĻ��ս�������ͼ����������ͼ�и���Ӧ��ǡ����ȫ��Ӧ����֪2Cu+O2+2H2SO4![]() 2CuSO4+2H2O����
2CuSO4+2H2O����

��1���������з�Ӧ�Ļ���������_____��Ӧ��
��2������2�к��еĽ�����_____���ѧʽ����
��3����������Ӧ�Ļ�ѧ����ʽΪ_____��
��4������Ni��Fe��Pd�Ļ����ǿ����������_____��
��5�����˻������÷Ͼɽ�������Լ�ͱ���������Դ�Ĵ�ʩ����_____��дһ������
���𰸡��û� Ni��Sn��Pb Fe+CuSO4��FeSO4+Cu Fe��Ni��Pd ��ֹ��������ʴ
��������
���������������ϡ����õ�����1����Һ1������1�м���ϡ���ᡢ���������¶ȼ��ȣ��õ��������ٺ���Һ3����Һ3�м������ۻ����ɺ�ɫ����A������A��ͭ������1�к����������١�ͭ����Һ3������ͭ����Һ4������������������������Ǧ����������֮ǰ����Һ1�м����õ���������������2����������2������������Ǧ��
��1�������������Hǰ��Ľ�����ϡ���ᷴӦ����H2����������������û���Ӧ��
��2������2�к��еĽ����ǣ�Ni��Sn��Pb��
��3������ݵķ�Ӧ����������ͭ��Ӧ��������������ͭ����ѧ����ʽΪ��Fe+CuSO4��FeSO4+Cu��
��4������Ni��Fe��Pd�Ļ����ǿ���������ǣ�Fe��Ni��Pd��
��5�����˻������÷Ͼɽ�������Լ�ͱ���������Դ�Ĵ�ʩ���з�ֹ��������ʴ��

 һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�
һŵ��ҵ�����ҵ���ּ�����������������ϵ�д�