��Ŀ����
����Ŀ��2016��1��29�ա��Ƽ��ձ�����������ѧ���״ν��ӿ�������Ķ�����̼����������ת��Ϊ�״���ͬʱ��ˮ���ɣ����о����ɳ�ȥ�����е��������������̼�����ɵļ״�������Ϊ���͵����ȼ�ϣ�������̼ת���ɼ״��Ĺ����У�һ���ؼ����������ҵ����ʵľ���������˴��о���Ա�������ڸ����²���ֽ�Ľ����ɴ������ȶ��Ժã����ظ�ʹ�ã�
��1��������̼ת���ɼ״�����ʾ��ͼ����ͼ��ʾ�� �ٴ˷�Ӧ�еĻ��������֣����������л���������� ��
����д����һ��Ӧ�Ļ�ѧ����ʽ �� 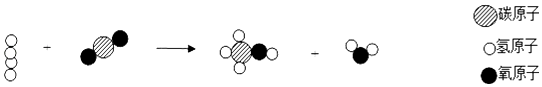
��2�����й��ڴ�����˵����ȷ���� �� ����������� ���ܸı仯ѧ��Ӧ�����ʣ��������������䣻�ڷ�Ӧ�����У����������ʲ��䣻�۷�Ӧ�����У������Ļ�ѧ���ʲ��䣻���ܼӿ컯ѧ��Ӧ�����ʣ��������������䣮
���𰸡�
��1��3���״���CO2+3H2 ![]() CH3OH+H2O
CH3OH+H2O
��2���٢�
���������⣺��1���۲���ʾ��ͼ��֪��Ӧ���������Ͷ�����̼���������Ǽ״���ˮ����˷�Ӧ�ķ���ʽΪ��CO2+3H2 ![]() CH3OH+H2O����Ӧ�еĻ�������3�֣����������л���������Ǽ״�����2���ɴ�����������֪�����ܸı仯ѧ��Ӧ�����ʣ��������������䣬˵����ȷ���ڷ�Ӧ�����У������Ļ�ѧ���ʲ��䣬˵�����۷�Ӧ�����У������Ļ�ѧ���ʲ��䣬˵����ȷ�����ܸı仯ѧ��Ӧ�����ʣ��������������䣬˵������ ���Դ��ǣ���1����3���״�����CO2+3H2
CH3OH+H2O����Ӧ�еĻ�������3�֣����������л���������Ǽ״�����2���ɴ�����������֪�����ܸı仯ѧ��Ӧ�����ʣ��������������䣬˵����ȷ���ڷ�Ӧ�����У������Ļ�ѧ���ʲ��䣬˵�����۷�Ӧ�����У������Ļ�ѧ���ʲ��䣬˵����ȷ�����ܸı仯ѧ��Ӧ�����ʣ��������������䣬˵������ ���Դ��ǣ���1����3���״�����CO2+3H2 ![]() CH3OH+H2O����2���٢ۣ�
CH3OH+H2O����2���٢ۣ�
�����㾫����ͨ��������ô������ص�������ú���д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ�����մ�������ý�����ڻ�ѧ��Ӧ���ܸı��������ʵĻ�ѧ��Ӧ���ʣ��������������ͻ�ѧ�����ڷ�Ӧǰ��û�з����仯�����ʣ���һ�������䣩�����ڻ�ѧ��Ӧ����������ýд����ã�ע�⣺a����ƽ b������ c�����ż����Խ����⣮

����Ŀ����ѧ��ȤС���ͬѧ��Э����ʦ����ʵ����ʱ������һƿ��ǩ��ҩƷ��ʴ��ȱ����ɫҺ�壮��ͼ��ʾ�����ǶԴ�ƿ��ɫҺ����ʲô��������Ȥ�����ǽ���������̽�����������̽���� 
��1������������衿 ����һ��ˮ
�������ϡ����
������������������Һ
��ȤС��ͬѧ�Ա�ǩ�ֽ�������ϸ�۲졢����������һ����Ϊ����һ����������������
��2������С��ͬѧ�Բ�����Ͳ���������������ʵ��̽��������Ʒ�����ʵ����֤��
���� | ��֤�ķ��� | ���� | ���� |
����� | ��ʢ���������Թ��м��������ĸ�Һ�壮 | ����������� | |
������ | ��ʢ���������Թ��м��������ĸ�Һ�壬���������ǵ�ľ�������Թܿڣ� | ���������� |
��3������չ��Ǩ�ơ�Ϊ�����ڽ���ʵ���г��ֱ�ǩ��ȱ����������㵹Һ�����ʱӦ��ע��������� ��