题目内容
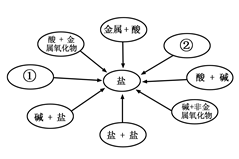
(2010·辽宁鞍山,20)小梅同学学习了单质、氧化物、酸、碱、盐性质后,发现许多不同类别的物质反应时能生成盐,于是她构建了甲图所示的知识网络图。
甲 乙
(1)请你把甲图中①②处补充完整,要求不能与图中已有信息重复。
① ;② 。
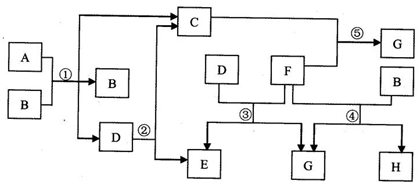
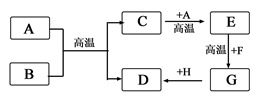
(2)乙图中A~H都是初中化学中常见的物质,已知A、B为黑色固体,D为红色固体单质,F为红色粉末,它们的转化关系如乙图所示。请回答:
①物质B的化学式为 。
②写出E+F→G的化学方程式 。
③写出一个能实现G+H→D的化学方程式 。


甲 乙
(1)请你把甲图中①②处补充完整,要求不能与图中已有信息重复。
① ;② 。
(2)乙图中A~H都是初中化学中常见的物质,已知A、B为黑色固体,D为红色固体单质,F为红色粉末,它们的转化关系如乙图所示。请回答:
①物质B的化学式为 。
②写出E+F→G的化学方程式 。
③写出一个能实现G+H→D的化学方程式 。
)(1)①酸+盐 ②金属+盐(①、②无顺序要求)
分析:本题难点主要是(1)问,可根据网络图已给信息,再结合单质、氧化物、酸、碱、盐之间相互反应生成盐的情况逐一核对可分析出.(2)本题解题的突破口是B为黑色固体,D、F为红色固体,首先确定这些常见的物质,再顺推或逆推即可解答本题.
解答:解:(1)、题中必须利用已有的网络图汇总信息,很明显给出的信息是在①处的左边是金属与酸的反应,那么金属与盐、与碱反应吗?可得出金属与盐可以发生置换反应生成盐,如Fe+CuSO4=FeSO4+Cu;在②的右下方是碱、盐与盐之间的反应,那么酸与盐可以反应生成盐吗?很明显可以,如H2SO4+BaCl2=BaSO4↓+2HCl;
故答案为:酸+盐;金属+盐
(2)B为黑色固体,D为红色固体,,可推知A、B为C和CuO,C为二氧化碳,D为Cu.
二氧化碳能与碳在高温下反应生成一氧化碳,故A是C,B为CuO,E为CO;初中另一常见的红色固体是Fe2O3,
实现转化④,可由铁与硫酸铜溶液反应.Fe+CuSO4═FeSO4+Cu
故答案为:(1)①酸+盐②金属+盐(①、②无顺序要求)
(2)①CuO
②Fe2O3+3CO 2Fe+3CO2;
2Fe+3CO2;
③Fe+CuSO4=Cu+FeSO4;
解答:解:(1)、题中必须利用已有的网络图汇总信息,很明显给出的信息是在①处的左边是金属与酸的反应,那么金属与盐、与碱反应吗?可得出金属与盐可以发生置换反应生成盐,如Fe+CuSO4=FeSO4+Cu;在②的右下方是碱、盐与盐之间的反应,那么酸与盐可以反应生成盐吗?很明显可以,如H2SO4+BaCl2=BaSO4↓+2HCl;
故答案为:酸+盐;金属+盐
(2)B为黑色固体,D为红色固体,,可推知A、B为C和CuO,C为二氧化碳,D为Cu.
二氧化碳能与碳在高温下反应生成一氧化碳,故A是C,B为CuO,E为CO;初中另一常见的红色固体是Fe2O3,
实现转化④,可由铁与硫酸铜溶液反应.Fe+CuSO4═FeSO4+Cu
故答案为:(1)①酸+盐②金属+盐(①、②无顺序要求)
(2)①CuO
②Fe2O3+3CO
 2Fe+3CO2;
2Fe+3CO2;③Fe+CuSO4=Cu+FeSO4;

练习册系列答案
 云南师大附小一线名师提优作业系列答案
云南师大附小一线名师提优作业系列答案 冲刺100分单元优化练考卷系列答案
冲刺100分单元优化练考卷系列答案
相关题目