��Ŀ����
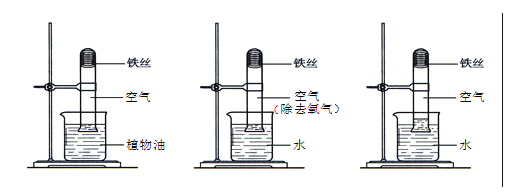
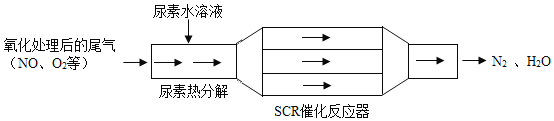
����Ŀ���ҹ����Ϳ�������SCR��������Ч���Ͳ��ͷ������ĵ�����������ŷš�SCR����ԭ������ͼ��
��1��NO2�е�Ԫ�صĻ��ϼ�Ϊ _______________��
��2������[CO(NH2)2]ˮ��Һ�ȷֽ�Ļ�ѧ����ʽΪ��CO(NH2)2+H2O![]() 2NH3+CO2��NH3��CO2��������Ϊ_______________________��
2NH3+CO2��NH3��CO2��������Ϊ_______________________��
��3��SCR����Ӧ����NH3��NO2��Ӧ�Ļ�ѧ����ʽΪ________________________________ ��
��4���ӱ��������ĽǶȷ��������Ϳ�������SCR�������ŵ�Ϊ__________________��
���𰸡�+4 17��22 8NH3+6NO2![]() 7N2+12H2O ���ٶ����������ŷţ���ֹ�����
7N2+12H2O ���ٶ����������ŷţ���ֹ�����
��������
��1��������������Ԫ�صĻ��ϼ�Ϊ-2,���ݻ�ѧʽ���������ϼ۴�����Ϊ��������Ԫ�صĻ��ϼ�Ϊ+4��
��2����ѧ����ʽ�����ʵ������ȵ��ڻ�ѧ����������Է�������֮�ȣ�������[CO(NH2)2]ˮ��Һ�ȷֽ���NH3��CO2��������=��2��17����44=17��22��
��3���������п�֪��SCR����Ӧ����NH3��NO2��Ӧ��������Ϊ������ˮ���仯ѧ����ʽΪ��8NH3+6NO2![]() 7N2+12H2O��
7N2+12H2O��
��4�����Ϳ�������SCR�����ɽ��͵�����������ŷţ����ٵ����������γ��������Ⱦ�����ȡ�