��Ŀ����
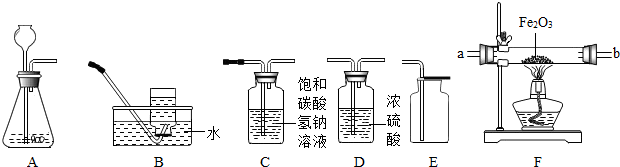
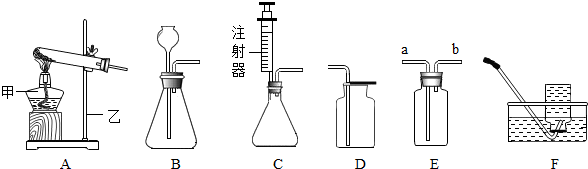
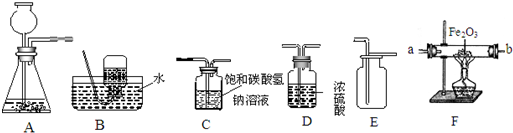
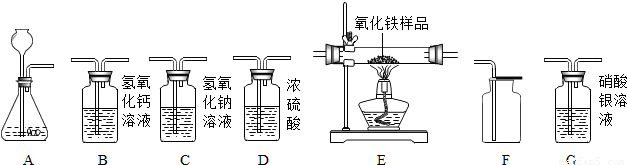
��ͼ��ʾΪʵ�����г����������Ʊ����������ռ�������ʵ��IJ���������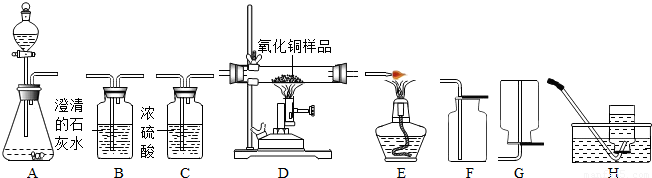
�Ը�����ĿҪ�ش��������⣺
��1�����Թ���������Һ�Ͷ�������Ϊԭ����ʵ�������Ʊ����ռ������������
����ѡ����������˳��Ϊ ����д���������ĸ����
����������ʱ����������Ӧ�Ļ�ѧ����ʽΪ�� ��
�����ӷ�Ӧ��ķ�Һ�л��ն������̿ɲ��õķ����� ��
��2���������ܶȺͿ������ƣ����ܽ���ˮ��ʵ�����ռ�������ѡ�õ�װ���� �������������ĸ����
��3��ijͬѧ�����ô�����CO�������ⶨij����ͭ��Ʒ�Ĵ��ȣ���Ʒ�е����ʲ��μӻ�ѧ��Ӧ��״̬Ҳ���ı䣩������֤��������������ʣ������Լ�����������
��������������˳��Ϊ��������CO����� ����д���������ĸ��
������ַ�Ӧ��Dװ�ò������еĹ��������3.2�ˣ������Ʒ������ͭ�������� �ˣ�
�۳����â������ݣ�������һװ��ǰ��������仯Ҳ�ܼ�����Ʒ������ͭ��������������������� ��
���𰸡���������1�����Թ���������Һ�Ͷ�������Ϊԭ����ʵ�������Ʊ����ռ����������������ʵ���Ŀ��ѡ����Ҫ���������ӣ�����ȡ�����ٸ���������Ȼ���������ſ������ռ������Ծݴ˽����⣻
�ڹ�������ֽ��ܹ�����ˮ������������д���÷�Ӧ�Ļ�ѧ����ʽ��
�۸��ݶ������̺�ˮ������ѡ����뷽����
��2��������Ϣ�ṩ�ĵ������ܶȺ��ܽ���ѡ���ռ�������
��3����һ����̼����������Ӧ���ɶ�����̼������ʵ��Ŀ��ѡ��װ�����ӣ�
�ڸ��ݹ�����ٵ���������ͭ����Ԫ�ص���������������ͭ��������
�ۿ�������װ��B�����ն�����̼������������ӣ�װ��B��Ӧǰ��������Ϊ��Ӧ���ɶ�����̼�����������ݷ�Ӧ�Ļ�ѧ����ʽ�����ö�����̼��������������ͭ��������
����⣺��1������ȡ����ѡ��ķ���װ����A����������Ӧ��ѡ��Ũ���ᣬȻ���������ſ������ռ���
����ȷ��˳��Ϊ��A��C��F��
�ڹ�������ֽ��ܹ�����ˮ���������ʸ÷�Ӧ�Ļ�ѧ����ʽΪ��2H2O2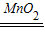 2H2O+O2����
2H2O+O2����
�ʴ�Ϊ��2H2O2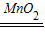 2H2O+O2����
2H2O+O2����
�۷�Ӧ��ķ�Һ�к��������ԵĶ������̺�ˮ���ʿ��Բ��ù��˷����룬�ٰ�������ɼ��ɣ�
�ʴ�Ϊ�����ˣ�
��2����Ϊ�������ܶȺͿ������ƣ����ܽ���ˮ����ֻ�ܲ�����ˮ���ռ���
�ʴ�Ϊ��H��
��3�������ô�����CO�������ⶨij����ͭ��Ʒ�Ĵ��ȣ���Ʒ�е����ʲ��μӻ�ѧ��Ӧ��״̬Ҳ���ı䣩����Ҫһ����̼��ԭ����ͭ��Dװ�ã�����֤���������������Ҫ�õ������ʯ��ˮ����������������˳��Ϊ��������CO�����D��B��
�ʴ�Ϊ��D��B��
�ڳ�ַ�Ӧ��Dװ�ò������еĹ��������3.2�ˣ����ٵ�������������ͭ����Ԫ�ص������������Ʒ������ͭ������Ϊ��3.2g÷�� ×100%��=16g��
×100%��=16g��
�ʴ�Ϊ��16��
���Ȳ����Ӧǰװ��B���������ٲ����Ӧ��װ��B�������������װ��B��������������յĶ�����̼���������ٸ���һ����̼������ͭ��Ӧ�Ļ�ѧ����ʽ�����ö�����̼��������������ͭ��������
�ʴ�Ϊ���Ȳ����Ӧǰװ��B���������ٲ����Ӧ��װ��B�������������װ��B��������������յĶ�����̼���������ٸ���һ����̼������ͭ��Ӧ�Ļ�ѧ����ʽ�����ö�����̼��������������ͭ��������
��������������Ҫ��������ʵ���Ŀ�Ľ����������ȡװ�ú��ռ�װ�á�����װ�õ�ѡ��ͬʱҲ�����˻�ѧ����ʽ����д���йصļ��㣬�ۺ��ԱȽ�ǿ�������òⶨ����ͭʧȥ����ʣ����������������ͭ������������õ�����ͭ������������Ҫ�����òⶨ�Ķ�����̼�������м����ȷ��ԭ������ڷ�Ӧ������������̼�Ƿ���������ȫ�����Լ��Ƿ������˿����еĶ�����̼��
�ڹ�������ֽ��ܹ�����ˮ������������д���÷�Ӧ�Ļ�ѧ����ʽ��
�۸��ݶ������̺�ˮ������ѡ����뷽����
��2��������Ϣ�ṩ�ĵ������ܶȺ��ܽ���ѡ���ռ�������
��3����һ����̼����������Ӧ���ɶ�����̼������ʵ��Ŀ��ѡ��װ�����ӣ�
�ڸ��ݹ�����ٵ���������ͭ����Ԫ�ص���������������ͭ��������
�ۿ�������װ��B�����ն�����̼������������ӣ�װ��B��Ӧǰ��������Ϊ��Ӧ���ɶ�����̼�����������ݷ�Ӧ�Ļ�ѧ����ʽ�����ö�����̼��������������ͭ��������
����⣺��1������ȡ����ѡ��ķ���װ����A����������Ӧ��ѡ��Ũ���ᣬȻ���������ſ������ռ���
����ȷ��˳��Ϊ��A��C��F��
�ڹ�������ֽ��ܹ�����ˮ���������ʸ÷�Ӧ�Ļ�ѧ����ʽΪ��2H2O2
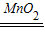 2H2O+O2����
2H2O+O2�����ʴ�Ϊ��2H2O2
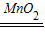 2H2O+O2����
2H2O+O2�����۷�Ӧ��ķ�Һ�к��������ԵĶ������̺�ˮ���ʿ��Բ��ù��˷����룬�ٰ�������ɼ��ɣ�
�ʴ�Ϊ�����ˣ�
��2����Ϊ�������ܶȺͿ������ƣ����ܽ���ˮ����ֻ�ܲ�����ˮ���ռ���
�ʴ�Ϊ��H��
��3�������ô�����CO�������ⶨij����ͭ��Ʒ�Ĵ��ȣ���Ʒ�е����ʲ��μӻ�ѧ��Ӧ��״̬Ҳ���ı䣩����Ҫһ����̼��ԭ����ͭ��Dװ�ã�����֤���������������Ҫ�õ������ʯ��ˮ����������������˳��Ϊ��������CO�����D��B��
�ʴ�Ϊ��D��B��
�ڳ�ַ�Ӧ��Dװ�ò������еĹ��������3.2�ˣ����ٵ�������������ͭ����Ԫ�ص������������Ʒ������ͭ������Ϊ��3.2g÷��
 ×100%��=16g��
×100%��=16g���ʴ�Ϊ��16��
���Ȳ����Ӧǰװ��B���������ٲ����Ӧ��װ��B�������������װ��B��������������յĶ�����̼���������ٸ���һ����̼������ͭ��Ӧ�Ļ�ѧ����ʽ�����ö�����̼��������������ͭ��������
�ʴ�Ϊ���Ȳ����Ӧǰװ��B���������ٲ����Ӧ��װ��B�������������װ��B��������������յĶ�����̼���������ٸ���һ����̼������ͭ��Ӧ�Ļ�ѧ����ʽ�����ö�����̼��������������ͭ��������
��������������Ҫ��������ʵ���Ŀ�Ľ����������ȡװ�ú��ռ�װ�á�����װ�õ�ѡ��ͬʱҲ�����˻�ѧ����ʽ����д���йصļ��㣬�ۺ��ԱȽ�ǿ�������òⶨ����ͭʧȥ����ʣ����������������ͭ������������õ�����ͭ������������Ҫ�����òⶨ�Ķ�����̼�������м����ȷ��ԭ������ڷ�Ӧ������������̼�Ƿ���������ȫ�����Լ��Ƿ������˿����еĶ�����̼��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ

 2Fe+3CO2��
2Fe+3CO2��