��Ŀ����
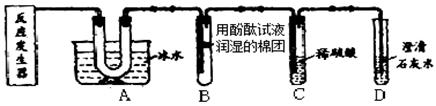
һ�Σ�С����ʵ���ҿ�������ͼ�龰��

�������뵽ҩƷ���ܱ����ˣ���ôNaOH��Һ�ڿ����б��ʵĻ�ѧ����ʽΪ______________________________________��
С������ʵ���ҵ������Լ����Ȼ�����Һ��ϡ���ᡢ��̪�Լ�������ƿ��NaOH��Һ��չ����̽����
����������
����٣�����������Һû�б���
����ڣ�____________________
����ۣ�����������Һ��ȫ����
��ʵ��̽����
����˼����С�շ��˷���II�Ľ��ۣ�������______________________________��
�۷�˼���ۣ�����������Һ��¶�ڿ��������ױ��ʣ���Ӧ____________���档

�������뵽ҩƷ���ܱ����ˣ���ôNaOH��Һ�ڿ����б��ʵĻ�ѧ����ʽΪ______________________________________��
С������ʵ���ҵ������Լ����Ȼ�����Һ��ϡ���ᡢ��̪�Լ�������ƿ��NaOH��Һ��չ����̽����
����������
����٣�����������Һû�б���
����ڣ�____________________
����ۣ�����������Һ��ȫ����
��ʵ��̽����
| �� �� | �� �� | �� �� | |
| I | ȡ������Һ���Թ��У���������__________�Լ� | ������������ | ����ٲ����� |
| II | ȡ������Һ���Թ��У��μӷ�̪�Լ� | ��Һ��� | ��Һ��һ����NaOH |
| III | a.ȡ������Һ���Թ��У��μ�����________�Լ��� | ____________ | ����ڳ��� |
| b.��a��������Һ�еμӷ�̪ | ��Һ��� | ||
�۷�˼���ۣ�����������Һ��¶�ڿ��������ױ��ʣ���Ӧ____________���档
(1) 2NaOH + CO2 = Na2CO3 + H2O
(2) ����������Һ���ֱ���
I������ III��CaCl2 ������ɫ����
Na2CO3��ҺҲ��ʹ��̪��� �ܷ�
(2) ����������Һ���ֱ���
I������ III��CaCl2 ������ɫ����
Na2CO3��ҺҲ��ʹ��̪��� �ܷ�
��������� NaOH��¶�ڿ����У���������еĶ�����̼��Ӧ������Na2CO3�����ʣ��ʱ��ʵĻ�ѧ����ʽΪ2NaOH + CO2 = Na2CO3 + H2O��
���������ݸ��ݲ���ٺ͢ۿ�֪�����ʵ����Ӧ�����֣���δ���ʣ����ֱ��ʺ�ȫ�����ʡ�
��ʵ��̽���ݸ�������ķ�����֪������NaOHû�б��ʣ���ȫ����NaOH������NaOH���ֱ��ʣ�����NaOH��Na2CO3�Ļ�������NaOH��ȫ���ʣ���ȫ����Na2CO3��
I������ʵ����ۡ�����ٲ�����������֪��Һ��һ������Na2CO3���ٽ��ʵ���������������ݡ�����֪����Һ�еμ���ϡ���ᡣ
II������Na2CO3�������Σ�������Һͬ��Ҳ�Լ��ԣ����׳ƴ������Ҳ��ʹ��̪��Һ��죬���Բ��ܵ����Է�̪�����ȷ����NaOH��
III������ʵ����ۡ�����ڳ���������֪��Һ��һ������Na2CO3��NaOH������������ķ�����֪Na2CO3��NaOH����Һ���ʼ��ԣ���������֤����ȥNa2CO3֮������֤NaOH����a����������Һ�еμ�������BaCl2����CaCl2����Һ�����ٲ���������������֤��Һ�к���Na2CO3��������ȫ��ȥ������е�Na2CO3��Ȼ���ٵμӷ�̪��Һ���ɼ���Һ��Ϊ��ɫ��֤������ڳ�����
�۷�˼���ۣ���������������Һ��¶�ڿ��������ױ��ʣ���Ӧ�ܷⱣ�档
������������֤��ʵ��̽����Ҫ������ʵ����ʻ�仯���ɣ����ݸ�����ʵ����Ʒ���������ʵ�顢������̽������ͨ���۲졢��¼�ͷ�����ʵ����������֤�����ʵ����ʻ�仯���ɵȡ�

��ϰ��ϵ�д�
 ���Ͱ�ͨ������ϵ�д�
���Ͱ�ͨ������ϵ�д� �ٷ�ѧ����ҵ��������ϵ�д�
�ٷ�ѧ����ҵ��������ϵ�д�
�����Ŀ

 ��
��