��Ŀ����
ʵ��С���о����ᡢ�������ơ����������������ʵĻ�ѧ���ʣ�������ͼ��ʾ��ʵ�顣��������ͼ������ҩƷ������һ������о���
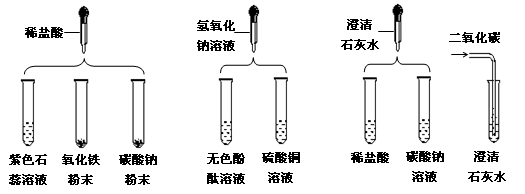
��1��ʵ���ij�Թ���Ϊ��ɫ��Һ�����Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ���ij�Թ���Ϊ��ɫ��Һ�������м��������� ����Һ��Ϊ��ɫ���ɴ��ƶϣ����Թ������ʢ�е������� ��
��3��ʵ���ij�Թܵײ�����ɫ��״���������������м��������� ���������ʧ��
��4��ʵ���ij�Թܵĵײ��а�ɫ���壬���˺�����Һ�еμ�ϡ���ᣬ��ʼʱ����������һ��ʱ��������ݳ��֡��ɴ��ƶϣ����Թ������������Ӧ�Ļ�ѧ����Ϊ ��
��5��ʵ���ij�Թ���ֻ�õ���ɫ��Һ�������м���������̼������Һ��ֻ�������ݡ���ô���Թ������������Ӧ�Ļ�ѧ����Ϊ ��
��6��ʵ�����ʱ�����ǽ�ʵ���Һ����ͬһ��Һ���У��۲�����Dz������յķ�Һ���ܳ����ԡ�Ϊ����֤���룬��������ʵ����δ�漰������Ļ�ѧ���ʣ���ѡҩƷ����ʵ�飺ȡ������Һ��Ʒ���Թ��У������м���һ������ ���۲�����֤�����յķ�Һ�����ԡ�ʵ���Ϊ�˱����Һ��ɲ������ �����ǶԷ�Һ������ǡ��������

��1��ʵ���ij�Թ���Ϊ��ɫ��Һ�����Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2��ʵ���ij�Թ���Ϊ��ɫ��Һ�������м��������� ����Һ��Ϊ��ɫ���ɴ��ƶϣ����Թ������ʢ�е������� ��
��3��ʵ���ij�Թܵײ�����ɫ��״���������������м��������� ���������ʧ��
��4��ʵ���ij�Թܵĵײ��а�ɫ���壬���˺�����Һ�еμ�ϡ���ᣬ��ʼʱ����������һ��ʱ��������ݳ��֡��ɴ��ƶϣ����Թ������������Ӧ�Ļ�ѧ����Ϊ ��
��5��ʵ���ij�Թ���ֻ�õ���ɫ��Һ�������м���������̼������Һ��ֻ�������ݡ���ô���Թ������������Ӧ�Ļ�ѧ����Ϊ ��
��6��ʵ�����ʱ�����ǽ�ʵ���Һ����ͬһ��Һ���У��۲�����Dz������յķ�Һ���ܳ����ԡ�Ϊ����֤���룬��������ʵ����δ�漰������Ļ�ѧ���ʣ���ѡҩƷ����ʵ�飺ȡ������Һ��Ʒ���Թ��У������м���һ������ ���۲�����֤�����յķ�Һ�����ԡ�ʵ���Ϊ�˱����Һ��ɲ������ �����ǶԷ�Һ������ǡ��������
��1��Fe2O3+6HCl=2FeCl3+3H2O ����2��ϡ���� �� ��ɫ��̪��Һ �� ��3��ϡ���
��4��Ca(OH)2+Na2CO3=CaCO3��+2NaOH����5��Na2CO3+2HCl=2NaCl+H2O+ CO2������6�����ۣ��𰸺������ɣ�
��4��Ca(OH)2+Na2CO3=CaCO3��+2NaOH����5��Na2CO3+2HCl=2NaCl+H2O+ CO2������6�����ۣ��𰸺������ɣ�
�����������1����������ϡ���ᷴӦ�����Ȼ�����ˮ���Ȼ�������ˮ�ʻ�ɫ��д����Ӧ����ʽ����2�������⡰��ɫ��Ϊ��ɫ���DZ�Ϊ��ɫ���ɵã�һ��������ɫ��̪��Һ�м����˼ʵ�������������£���3������ɫ��״������Ϊ������ͭ��������������У��ʼ��������м���������ϡ���ᣬ��ʹ������ʧ����4�����ײ��а�ɫ���壬��ʼʱ����������һ��ʱ��������ݳ��֡���˵����Һ����̼������ڣ���ʼʱ��������Һ�е���һ�����ʷ�Ӧ�������Ϊ����������̼���Ƶķ�Ӧ����5��������������̼������Һ��ֻ�������ݡ���˵��������ڣ���Ϊ������̼���Ƶķ�Ӧ����6������ʵ�����漰���������У����ָʾ�����ᡢ��ķ�Ӧ������������������Σ�û���漰��������ķ�Ӧ��ѡ�ó����Ļ��ý������ɡ�

��ϰ��ϵ�д�
�����Ŀ



 �� P��ֵΪ___________��
�� P��ֵΪ___________��