��Ŀ����
��ʯ���dz��õĸ����?һ�������������ƺ��������ƳɵĹ��������ʵ��С����ȷ��ʵ����ij���ü�ʯ�ҵijɷֽ���������̽����
�����롿���ü�ʯ���п��ܺ��������ơ��������ơ�̼���ơ�̼��ơ��������ƣ�
�����ϡ�һ������£��������ˮ��Һ�вſ������Ӧ��
��ʵ�顿ȡ��ʯ����Ʒ����������ˮ�в����Թ���������˺�����Һ�еμӳ���ʯ��ˮ����Һ����ǣ�
��1����ͬѧ������Ʒ�м���ˮ�����ڷ��ȣ����ƶ���Ʒ��һ�����������ƣ�������������Ƿ���ȷ����˵�����ɣ�
��2���ü�ʯ����һ�����еijɷ��� ��
��3����ˮʱ���ֵIJ����Թ����� ����Դ���������?�ֱ�Ϊ �� �� ��
�����롿���ü�ʯ���п��ܺ��������ơ��������ơ�̼���ơ�̼��ơ��������ƣ�
�����ϡ�һ������£��������ˮ��Һ�вſ������Ӧ��
��ʵ�顿ȡ��ʯ����Ʒ����������ˮ�в����Թ���������˺�����Һ�еμӳ���ʯ��ˮ����Һ����ǣ�
��1����ͬѧ������Ʒ�м���ˮ�����ڷ��ȣ����ƶ���Ʒ��һ�����������ƣ�������������Ƿ���ȷ����˵�����ɣ�
��2���ü�ʯ����һ�����еijɷ���
��3����ˮʱ���ֵIJ����Թ�����
��������1�������������Ƽ�������ˮ���ܽ�Ҳ�ų������Ƚ��з�����
��2����������Һ�еμӳ���ʯ��ˮ����Һ����ǣ���Ϊֻ��̼���������������Ʒ�Ӧ���ɰ�ɫ��������Һ���ܱ���Ǽ��Է�����
��3������̼���������ˮ������̼��ƿ������Ա��ʵģ�Ҳ�������Թ��������е���������ˮ��Ӧ���ɵ��������ƣ����ɵ����������������е�̼���Ʒ�Ӧ����̼��ƺ��������ƣ�Ҳ�����ǹ��������е��������ƺ�̼���Ʒ�Ӧ����̼��ƺ��������ƣ�
��2����������Һ�еμӳ���ʯ��ˮ����Һ����ǣ���Ϊֻ��̼���������������Ʒ�Ӧ���ɰ�ɫ��������Һ���ܱ���Ǽ��Է�����
��3������̼���������ˮ������̼��ƿ������Ա��ʵģ�Ҳ�������Թ��������е���������ˮ��Ӧ���ɵ��������ƣ����ɵ����������������е�̼���Ʒ�Ӧ����̼��ƺ��������ƣ�Ҳ�����ǹ��������е��������ƺ�̼���Ʒ�Ӧ����̼��ƺ��������ƣ�
����⣺��1����Ϊ�������Ƽ�������ˮ���ܽ�Ҳ�ų������ȣ����Բ����ɴ��ƶ���Ʒ��һ�����������ƣ�
��2����������Һ�еμӳ���ʯ��ˮ����Һ����ǣ�˵����Һ�к���̼���ƣ���Ϊ����������ֻ��̼���������������Ʒ�Ӧ���ɰ�ɫ��������Һ���ܱ���ǣ���̼��������������������еĶ�����̼���ɵģ�
��3������̼���������ˮ����˲����Թ�����̼��ƣ�̼��Ƶ���Դ�������ǹ��������б�������̼��ƣ�Ҳ�����ǹ��������е���������ˮ��Ӧ���ɵ��������ƣ����ɵ����������������е�̼���Ʒ�Ӧ����̼��ƺ��������ƣ�Ҳ�����ǹ��������е��������ƺ�̼���Ʒ�Ӧ����̼��ƺ��������ƣ�
�ʴ�Ϊ����1������ȷ?��ΪNaOH�ܽ���ˮҲ���Է��ȣ���2��̼���ƣ�
��3��̼��ƣ� ���������б�������̼��ƣ����������е���������ˮ��Ӧ���ɵ��������ƣ����ɵ����������������е�̼���Ʒ�Ӧ����̼��ƺ��������ƣ�?���������е��������ƺ�̼���Ʒ�Ӧ����̼��ƺ��������ƣ�
��2����������Һ�еμӳ���ʯ��ˮ����Һ����ǣ�˵����Һ�к���̼���ƣ���Ϊ����������ֻ��̼���������������Ʒ�Ӧ���ɰ�ɫ��������Һ���ܱ���ǣ���̼��������������������еĶ�����̼���ɵģ�
��3������̼���������ˮ����˲����Թ�����̼��ƣ�̼��Ƶ���Դ�������ǹ��������б�������̼��ƣ�Ҳ�����ǹ��������е���������ˮ��Ӧ���ɵ��������ƣ����ɵ����������������е�̼���Ʒ�Ӧ����̼��ƺ��������ƣ�Ҳ�����ǹ��������е��������ƺ�̼���Ʒ�Ӧ����̼��ƺ��������ƣ�
�ʴ�Ϊ����1������ȷ?��ΪNaOH�ܽ���ˮҲ���Է��ȣ���2��̼���ƣ�
��3��̼��ƣ� ���������б�������̼��ƣ����������е���������ˮ��Ӧ���ɵ��������ƣ����ɵ����������������е�̼���Ʒ�Ӧ����̼��ƺ��������ƣ�?���������е��������ƺ�̼���Ʒ�Ӧ����̼��ƺ��������ƣ�
����������ͨ��ʵ���ҳ����������ʯ���Ƿ���ʵ�ʵ��̽�����ۺϿ����������ơ���ʯ�ҡ�̼���Ƶȵ����ʣ��Լ���ˮ�������֮��Ļ�ѧ��Ӧ���ѶȽϴ�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
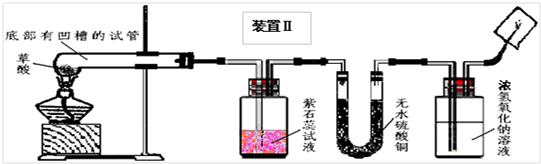
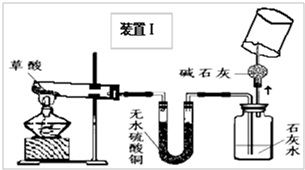 ��2008?��ɽ��һģ��ij��ѧ��ѧѧϰС�����Ķ��ˡ���ȥ֯�������յķ��������ֲ����ܹ����ڳ�ȥˮ���ա�Ѫ�ա����⡢��īˮ�����գ��Բ��������Ũ�����Ȥ����ʦ����ͬѧ�Dz���Ļ�ѧʽΪH2C2O4�����ȷֽ⣮ͬѧ�ǶԲ������ȷֽ����̽����
��2008?��ɽ��һģ��ij��ѧ��ѧѧϰС�����Ķ��ˡ���ȥ֯�������յķ��������ֲ����ܹ����ڳ�ȥˮ���ա�Ѫ�ա����⡢��īˮ�����գ��Բ��������Ũ�����Ȥ����ʦ����ͬѧ�Dz���Ļ�ѧʽΪH2C2O4�����ȷֽ⣮ͬѧ�ǶԲ������ȷֽ����̽����