��Ŀ����
 ��2008?��ɽ��һģ��ij��ѧ��ѧѧϰС�����Ķ��ˡ���ȥ֯�������յķ��������ֲ����ܹ����ڳ�ȥˮ���ա�Ѫ�ա����⡢��īˮ�����գ��Բ��������Ũ�����Ȥ����ʦ����ͬѧ�Dz���Ļ�ѧʽΪH2C2O4�����ȷֽ⣮ͬѧ�ǶԲ������ȷֽ����̽����
��2008?��ɽ��һģ��ij��ѧ��ѧѧϰС�����Ķ��ˡ���ȥ֯�������յķ��������ֲ����ܹ����ڳ�ȥˮ���ա�Ѫ�ա����⡢��īˮ�����գ��Բ��������Ũ�����Ȥ����ʦ����ͬѧ�Dz���Ļ�ѧʽΪH2C2O4�����ȷֽ⣮ͬѧ�ǶԲ������ȷֽ����̽������1��������⣺�������ȷֽ�IJ������ʲô�أ�
��2���������ϣ��ٲ��ᾧ���۵�ϵͣ����ȵ�182��㿪ʼ�ۻ���
����ˮ����ͭΪ��ɫ���壬������ˮ�����ɫ���壬�����ڼ���ˮ�����������
�ۼ�ʯ�����������ƺ����������ֹ���Ļ����dz��õĸ��������¶�ڿ����������������ˮ��Ӧ����ѧ����ʽΪ
CaO+H2O=Ca��OH��2
CaO+H2O=Ca��OH��2
���������������������̼��Ӧ����ѧ����ʽΪ2NaOH+CO2�TNa2CO3+H2O
2NaOH+CO2�TNa2CO3+H2O
����3����������裺
��һ�֣�����ֽ������CO2��H2��
�ڶ��֣�����ֽ������CO2��CO��H2O��
�����֣�����ֽ������CO2��H2O��
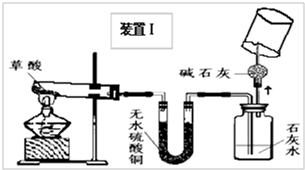
��4��ʵ��װ�õ���Ƽ�ʵ�飺
�ס���ͬѧ�ֱ������װ�â��װ�â��ֱ��ø��Ե�װ�ý�����ʵ�飮
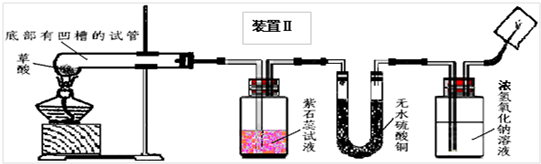
��5��ʵ���¼��
��ͬѧʵ��۲쵽��ˮ����ͭ�ɰ�ɫ�����ɫ������ʯ��ˮ����ǣ������ɵ�����ͨ����ʯ�Һ��ڼ��촦��ȼ����ȼ�գ��ڻ����Ϸ���һ���ڱڸ��г���ʯ��ˮ���ձ��������ʯ��ˮ����ǣ�
��ͬѧʵ��۲쵽��ʯ����Һ��죬��ˮ����ͭ�ɰ�ɫ�����ɫ�������ɵ�����ͨ������������Һ���ڼ��촦��ȼ����ȼ�գ��ڻ����Ϸ���һ���ڱڸ��г���ʯ��ˮ���ձ��������ʯ��ˮ����ǣ�
��6�����ۣ���2�ֲ�����ϲ���ֽ�ʵ�ʣ��������ȷֽ�Ļ�ѧ��Ӧ����ʽΪ��
H2C2O4
CO��+CO2��+H2O
| ||
H2C2O4
CO��+CO2��+H2O
��
| ||
��7����˼�����ۣ�ʵ��֮�ס���ͬѧ��������Ե���ƣ����ҵ��˲��㣮
A���ڲ������ȷֽ�װ������ϸ���ѧ����װ��
��
��
�������B���ڼ���ֽ�����Ƿ����ˮ������ϣ���©������װ��
��
��
�������C���ڼ���ֽ�����Ƿ���������������ϣ�������Ϊ���������ˣ���ͬʱ��ΪֻҪʹ����
��װ��
��
��
�Ϳ�˳�����ʵ��֤����������������ڻ�������һ�����ձ����۲죬���ڱ���ˮ���γ����У���֮û��
�ڻ�������һ�����ձ����۲죬���ڱ���ˮ���γ����У���֮û��
����������2���������ƺ�ˮ��Ӧ�������������ƣ����������ƺͶ�����̼��Ӧ������̼���ƺ�ˮ�����Ծݴ���ɽ��
��6��������2��������˵������ֽ�����˶�����̼��һ����̼��ˮ�����Ծݴ�д����ѧ����ʽ��
��7��A�����ݲ�����۵㼴����װ���еļ���װ�ÿ�������жϣ�
B���ڼ���ˮ������ʱ��Ϊ�˷�ֹͨ������Һ���������ţ�����Ӧ���ȼ��飬���Ծݴ˷�����
C����������ȼ�յIJ��T�����ļ��鷽�������н��
��6��������2��������˵������ֽ�����˶�����̼��һ����̼��ˮ�����Ծݴ�д����ѧ����ʽ��
��7��A�����ݲ�����۵㼴����װ���еļ���װ�ÿ�������жϣ�
B���ڼ���ˮ������ʱ��Ϊ�˷�ֹͨ������Һ���������ţ�����Ӧ���ȼ��飬���Ծݴ˷�����
C����������ȼ�յIJ��T�����ļ��鷽�������н��
����⣺��2���������ƺ�ˮ��Ӧ�������������ƣ��仯ѧ����ʽΪ��CaO+H2O�TCa��OH��2�����������ƺͶ�����̼��Ӧ������̼���ƺ�ˮ���仯ѧ����ʽΪ��2NaOH+CO2=Na2CO3+H2O��
��6��������2��������˵������ֽ�����˶�����̼��һ����̼��ˮ�����Ծݴ�д����ѧ����ʽΪ��H2C2O4
CO��+CO2��+H2O��
��7��A�������������Ϣ����֪��������۵�ϵͣ�����Ϊ��ֹ�����ۻ������뵼����Ӱ��ʵ�飬����װ�â���ѡ��ĵײ��а��۵��ԹܽϺã�
B���ڼ���ˮ������ʱ��Ϊ�˷�ֹͨ������Һ���������ţ�����Ӧ���ȼ��飬����װ�â��ܹ������ˮ�����ɣ���װ�â�ͨ������ɫʯ����Һ�������ˮ�ļ���
C������ȼ��Ҫ����ˮ������β��ȼ�ղ�����ˮ�������жϣ���������ɫʯ����Һ����װ�â���ɶ��Ƿ�����������жϣ�����ķ���Ϊ���ڻ�������һ�����ձ����۲죬���ڱ���ˮ���γ����У���֮û�У�
�ʴ�Ϊ����2��CaO+H2O=Ca��OH��2��2NaOH+CO2�TNa2CO3+H2O��
��6��H2C2O4
CO��+CO2��+H2O��
��7��A����
B����
C�����ڻ�������һ�����ձ����۲죬���ڱ���ˮ���γ����У���֮û�У�
��6��������2��������˵������ֽ�����˶�����̼��һ����̼��ˮ�����Ծݴ�д����ѧ����ʽΪ��H2C2O4
| ||
��7��A�������������Ϣ����֪��������۵�ϵͣ�����Ϊ��ֹ�����ۻ������뵼����Ӱ��ʵ�飬����װ�â���ѡ��ĵײ��а��۵��ԹܽϺã�
B���ڼ���ˮ������ʱ��Ϊ�˷�ֹͨ������Һ���������ţ�����Ӧ���ȼ��飬����װ�â��ܹ������ˮ�����ɣ���װ�â�ͨ������ɫʯ����Һ�������ˮ�ļ���
C������ȼ��Ҫ����ˮ������β��ȼ�ղ�����ˮ�������жϣ���������ɫʯ����Һ����װ�â���ɶ��Ƿ�����������жϣ�����ķ���Ϊ���ڻ�������һ�����ձ����۲죬���ڱ���ˮ���γ����У���֮û�У�
�ʴ�Ϊ����2��CaO+H2O=Ca��OH��2��2NaOH+CO2�TNa2CO3+H2O��
��6��H2C2O4
| ||
��7��A����
B����
C�����ڻ�������һ�����ձ����۲죬���ڱ���ˮ���γ����У���֮û�У�
�������������ճ�����������ʣ��������������ʶ������м��顢�����������DZ������յĻ������ܣ�Ҳ�ǻ�ѧʵ�鿼����ȵ�֮һ��

��ϰ��ϵ�д�
�����Ŀ
 ��2008?��ɽ��һģ�������������������ⶨ��ʵ��װ����ͼ������˵����ȷ���ǣ�������
��2008?��ɽ��һģ�������������������ⶨ��ʵ��װ����ͼ������˵����ȷ���ǣ�������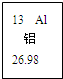 ��2008?��ɽ��һģ����ͼ��Ԫ�����ڱ��е�һ��Ԫ�أ������йظ�Ԫ�ص���Ϣ��ȷ���ǣ�������
��2008?��ɽ��һģ����ͼ��Ԫ�����ڱ��е�һ��Ԫ�أ������йظ�Ԫ�ص���Ϣ��ȷ���ǣ�������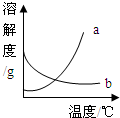 ��2008?��ɽ��һģ����ͼ��a��b�������ʵ��ܽ�����ߣ�����ʱ����ʢ��a��b������Һ���Թֱܷ�����ձ��ڴ������µ�ˮ�У������ձ��ڵ�ˮ�м�������粒����Ũ������Թ�����ʾ������ȷ���� ��������
��2008?��ɽ��һģ����ͼ��a��b�������ʵ��ܽ�����ߣ�����ʱ����ʢ��a��b������Һ���Թֱܷ�����ձ��ڴ������µ�ˮ�У������ձ��ڵ�ˮ�м�������粒����Ũ������Թ�����ʾ������ȷ���� ��������