��Ŀ����
ij����С��ͨ��Internet�������ϵ�֪�������Ķ�ӰҺ�к���һ������AgNO3�����Ǵ���Ӱ���ռ���һЩ�����Ķ�ӰҺ���������е����Ե��ʵ���ʽȫ�����գ����ǣ����ǽ��������µ�ʵ������������
�ٷ�����ӰҺ
| ||
| ����a |
�ڽ��������
| ||
| ����b |
��ش�
��1������a��b��ͬ����Ҫ�õ��IJ��������У��ձ�����������
��2����ͬѧ��Ϊ����ӰҺ�м��������ͭ�ۣ��õ��Ľ�������ﺬ������ͭ����ͬѧ��Ϊ����ӰҺ�м�����������ۣ���õ��Ľ�������ﺬ��������������Ϊ
��3����ȥ�����л��еĽ��������������������м����Լ�ʱ��Ӧ�Ļ�ѧ����ʽΪ
��4����ҵ�������Ľ���һ�㺬�����ʣ�ijͬѧȡ�����ھƾ��ƻ��������գ������ڱ�Ϳ�г���ʯ��ˮ���ձ�����ȼ�ճ��Ϸ�������ʯ��ˮ����ǣ�˵�������к�������̼����ͬѧ��˵��
��������1�����ݹ���װ��������������н��
��2�����ݽ������˳���ǿ�Ľ����ܽ�������Ľ�����������Һ���û�������������֮ǰ�Ľ��������ᷴӦ�û����������ӷ����IJ������㷽��Է����������ۣ�
��3������������֮ǰ�Ľ��������ᷴӦ�û���������ѡȡһ���Ὣ���۳�ȥ��
��4������Ԫ���غ����ж�ʹʯ��ˮ����ǵĶ�����̼����Դ��
��2�����ݽ������˳���ǿ�Ľ����ܽ�������Ľ�����������Һ���û�������������֮ǰ�Ľ��������ᷴӦ�û����������ӷ����IJ������㷽��Է����������ۣ�
��3������������֮ǰ�Ľ��������ᷴӦ�û���������ѡȡһ���Ὣ���۳�ȥ��
��4������Ԫ���غ����ж�ʹʯ��ˮ����ǵĶ�����̼����Դ��
����⣺��1������װ��������������ձ�����������©��������̨���������ڲ������������ձ�����������©����
��2��ʹ�������û���ӰҺ�е��������������ۿɼ������ϡ�������ȥ����ʹ��ͭ�۽����û�����ͭ�Ļ�Խ���������������ʷ�Ӧ���������������룮�ҷ����Ļ�ѧ����ʽΪFe+2AgNO3=Fe��NO3��2+2Ag��
��3����Ϊ���������ǰ�棬��������ĺ��棬���Կ���һ���������Ὣ����ȥ����Ӧ�Ļ�ѧ����ʽΪ��Fe+H2SO4=FeSO4+H2����
��4��ʹʯ��ˮ����ǵĶ�����̼������������Ͻ��е�̼ȼ�����ɵĶ�����̼��Ҳ�����Ǿƾ�ȼ�����ɵĶ�����̼�����Բ�����������������˵�������к�������̼��
�ʴ�Ϊ����1��©����
��2���ң� ����ͭ���ã������׳�ȥ��Fe+2AgNO3=Fe��NO3��2+2Ag��
��3��Fe+H2SO4=FeSO4+H2����
��4������ȷ��
��2��ʹ�������û���ӰҺ�е��������������ۿɼ������ϡ�������ȥ����ʹ��ͭ�۽����û�����ͭ�Ļ�Խ���������������ʷ�Ӧ���������������룮�ҷ����Ļ�ѧ����ʽΪFe+2AgNO3=Fe��NO3��2+2Ag��
��3����Ϊ���������ǰ�棬��������ĺ��棬���Կ���һ���������Ὣ����ȥ����Ӧ�Ļ�ѧ����ʽΪ��Fe+H2SO4=FeSO4+H2����
��4��ʹʯ��ˮ����ǵĶ�����̼������������Ͻ��е�̼ȼ�����ɵĶ�����̼��Ҳ�����Ǿƾ�ȼ�����ɵĶ�����̼�����Բ�����������������˵�������к�������̼��
�ʴ�Ϊ����1��©����
��2���ң� ����ͭ���ã������׳�ȥ��Fe+2AgNO3=Fe��NO3��2+2Ag��
��3��Fe+H2SO4=FeSO4+H2����
��4������ȷ��
���������⿼���˽������˳��Ӧ�á��û���Ӧ�����˲�������������ķ�Ӧ�Լ�ʵ�鷽���ıȽϣ�������Ϲ㣬�����ѣ�

��ϰ��ϵ�д�
�����Ŀ
���������Ǵ�����Ⱦ��֮һ����һΪ�ҹ����������������ж�ÿ�ο��������ⶨ�е�S02���Ũ����ֵ��

| S02���Ũ����ֵ����λmg?m-3�� | ||
| һ���� | ������ | ������ |
| 0.15 | 0.50 | 0.70 |
��1�����װ�õ�������ʱ�������Թ���װ��������ˮ����֤�������ܵ��¶˽�û��ˮ�У���Ȼ��������������ע�����Ļ���ʱ�����ῴ��________ ����дʵ��������֤����װ�õ����������ã�
��2����֪�����������ˮ�ķ�ӦΪ��SO2+I2+2H2O�TH2SO4+2HI�����Թ��м���1.0mL������������Ϊ1.27��10-4�ĵ�ˮ�� ��ʱ��Һ���ܶ���ˮ���ܶȽ��� ����������������ˮϡ�ͺ��ټ���2��3����ɫ������Һ�������ⵥ�ʱ���ɫ�������Ƴ���ҺA���ⶨָ���ص�Ŀ�����SO2�ĺ���ʱ������ע�����Ļ�������������A��Һ��________ɫ��Ϊ________ɫʱ��Ӧǡ����ȫ���У�
��3������С��ֳɵ�һС��͵ڶ�С�飬ʹ����ͬ��ʵ��װ�ú���ҺA���ֱ������ѧʵ���Һͽ��ҵĿ����е�S02����������Ӧǡ����ȫ���У���¼�����������£�����ÿ�γ���500mL�����뽫������д����������ʱ������λ��Ч���֣�����I-127��S-32��O-16��
��������ͬ���IJ���ֵ
| �顡�� | ��һС�飨ʵ���ң� | �ڶ�С�飨���ң� |
| �������� | 110 | 150 |
| ������S02�ĺ�������λ��mg?m-3�� |
���������Ǵ�����Ⱦ��֮һ����һΪ�ҹ����������������ж�ÿ�ο��������ⶨ�е�S02���Ũ����ֵ��

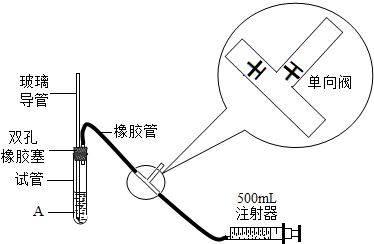
���һ����ֹܾ涨��2005��1��1������ʵ�������뻷����ܷ�Χ��Ϊ���Եزⶨ��Χ�����е�SO2������ij����С�����������ͼ��ʵ��װ�ã�
��1�����װ�õ�������ʱ�������Թ���װ��������ˮ����֤�������ܵ��¶˽�û��ˮ�У���Ȼ��������������ע�����Ļ���ʱ�����ῴ��______ ����дʵ��������֤����װ�õ����������ã�
��2����֪�����������ˮ�ķ�ӦΪ��SO2+I2+2H2O�TH2SO4+2HI�����Թ��м���1.0mL������������Ϊ1.27×10-4�ĵ�ˮ�� ��ʱ��Һ���ܶ���ˮ���ܶȽ��� ����������������ˮϡ�ͺ��ټ���2��3����ɫ������Һ�������ⵥ�ʱ���ɫ�������Ƴ���ҺA���ⶨָ���ص�Ŀ�����SO2�ĺ���ʱ������ע�����Ļ�������������A��Һ��______ɫ��Ϊ______ɫʱ��Ӧǡ����ȫ���У�
��3������С��ֳɵ�һС��͵ڶ�С�飬ʹ����ͬ��ʵ��װ�ú���ҺA���ֱ������ѧʵ���Һͽ��ҵĿ����е�S02����������Ӧǡ����ȫ���У���¼�����������£�����ÿ�γ���500mL�����뽫������д����������ʱ������λ��Ч���֣�����I-127 S-32 O-16��
���� ��ͬ���IJ���ֵ
��4��ͨ���ⶨ�����㣬���жϳ����⻯ѧʵ���ҵĿ�����S02�ĺ�������______�������һ�����оٵĵȼ�����

| S02���Ũ����ֵ | ||
| һ���� | ������ | ������ |
| 0.15 | 0.50 | 0.70 |
��1�����װ�õ�������ʱ�������Թ���װ��������ˮ����֤�������ܵ��¶˽�û��ˮ�У���Ȼ��������������ע�����Ļ���ʱ�����ῴ��______ ����дʵ��������֤����װ�õ����������ã�
��2����֪�����������ˮ�ķ�ӦΪ��SO2+I2+2H2O�TH2SO4+2HI�����Թ��м���1.0mL������������Ϊ1.27×10-4�ĵ�ˮ�� ��ʱ��Һ���ܶ���ˮ���ܶȽ��� ����������������ˮϡ�ͺ��ټ���2��3����ɫ������Һ�������ⵥ�ʱ���ɫ�������Ƴ���ҺA���ⶨָ���ص�Ŀ�����SO2�ĺ���ʱ������ע�����Ļ�������������A��Һ��______ɫ��Ϊ______ɫʱ��Ӧǡ����ȫ���У�
��3������С��ֳɵ�һС��͵ڶ�С�飬ʹ����ͬ��ʵ��װ�ú���ҺA���ֱ������ѧʵ���Һͽ��ҵĿ����е�S02����������Ӧǡ����ȫ���У���¼�����������£�����ÿ�γ���500mL�����뽫������д����������ʱ������λ��Ч���֣�����I-127 S-32 O-16��
���� ��ͬ���IJ���ֵ
| �� �� | ��һС�飨ʵ���ң� | �ڶ�С�飨���ң� |
| �������� | 110 | 150 |
| ������S02��� |
 ��ͨ���������������彻��������������ų�������̼���������ų��Ķ�����̼���������Կ����أ����������л���ò������أ�ijѧУѧ������С���������ͼ��ʾװ�ý���̽����
��ͨ���������������彻��������������ų�������̼���������ų��Ķ�����̼���������Կ����أ����������л���ò������أ�ijѧУѧ������С���������ͼ��ʾװ�ý���̽����