��Ŀ����
ij�о���ѧϰС������˲ⶨ���Na2CO3����Ʒ��ֻ��NaCl���ʣ���Na2CO3����������ʵ�鷽������ش��������⣺
С���ϣ���ʯ�ҵ���Ҫ�ɷ����������ƺ������ƵĻ����
��1�����巨��
��ԭ��˼·������Na2CO3��ϡ���ᷴӦ����CO2��ͨ��������װ��ʵ��ǰ��������ó�CO2�������Ӷ�����̼���Ƶ�������������������ԭװ���ڿ�����Ӱ�죩
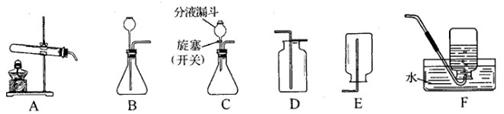
��ʵ�������С��ͬѧ��������˼·���������ͼ1��ʵ��װ�á�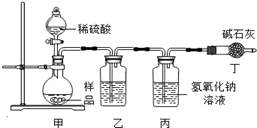
ͼ1
��ʵ��װ������ʢ�ŵ��Լ�Ϊ�� ����
�������� ��
װ�ö��м�ʯ�ҵ��������� ��������ĸ��
A������Na2CO3��ϡ���ᷴӦ����CO2
B����ֹ�������ж�����̼�������
C�����������̼
��ʵ��������μ�ϡ������ٶȹ��죬�ᵼ�²����Ʒ��̼���Ƶ�
���������� ������д��ƫ�ߡ���ƫ�͡����䡱����
��С��ͬѧ��ΪС�Ƶķ����������⣬��ʹ�����淶��Ҳ����ɽ��ƫ�ͣ���
��Ϊ���������� ��Ӧ�ý�װ�ü���˫����
������������������������ͼ2װ�ã�ʵ�������ͨ��һ��ʱ�������
Ŀ���� ������NaOH��Һ�������� ��
ͼ2
��2����������
��ʵ��ԭ��������̼�������������ӽ�����ɳ�����ͨ���������������ó�̼���Ƶ�����������
��ʵ�鲽�衿����������ƽ��ȡ��Ʒ11��0g�����ձ��У���ˮʹ����ȫ�ܽ⣻
�ڼ��������CaCl2��Һ��ַ�Ӧ��֤��CaCl2��������Na2CO3����ȫ��Ӧ���ķ�����: ���ã����ϲ���Һ�еμ� ��Һ�����û�а�ɫ�������ɣ�֤��CaCl2������
�۹��ˡ�����X�������������������Ϊ10��0g������X�������� �����ȱ�ٴ˲��������̼������������ ����д��ƫ�ߡ���ƫ�͡����䡱����
��ͨ�������ṩ�����ݼ������Ʒ��̼���Ƶ���������Ϊ�� ����д��������̣����������0��1%����3�֣�
��1����Ũ���ᣨ��ŨH2SO4�� ����ˮ�����������CO2�� B
��ƫ�� ����CO2��������ƿ�� ��������CO2ȫ�����뵽��װ���У�ʹ����ȫ������������Һ���� ���տ����е�CO2
��2����CaCl2 �� ϴ�� ƫ�� �� 96��4%
���������������1����Ϊ��ͨ��������װ��ʵ��ǰ��������ó�CO2���������Զ�����̼����������dz�ȷ�����Եñ�֤���뵽װ�ñ����Ǵ����Ķ�����̼�����Ԣ�ʵ��װ������ʢ�ŵ��Լ�Ϊ��Ũ���ᣨ��ŨH2SO4���������ǣ�����ˮ�����������CO2����װ�ö��м�ʯ�ҵ������ǣ���ֹ�������ж�����̼������У���ѡB����ʵ��������μ�ϡ������ٶȹ��죬�����Ķ�����̼��������Ӧ�����ų���װ�ñ��⣬ʹ�ö�����̼������ƫС����Ȼ�ᵼ�²����Ʒ��̼���Ƶ���������ƫ�ͣ���С��ͬѧ��ΪС�Ƶķ����������⣬��ʹ�����淶��Ҳ����ɽ��ƫ�ͣ����������ǣ���CO2��������ƿ�ڣ�Ӧ�ý�װ�ü���˫��������������������������ͼ2װ�ã�ʵ�������ͨ��һ��ʱ�������Ŀ���ǣ���������CO2ȫ�����뵽��װ���У�ʹ����ȫ������������Һ���գ�����NaOH��Һ�������ǣ����տ����е�CO2����ֹʹ������̼������ƫ��
��2���ڼ��������CaCl2��Һ��ַ�Ӧ��֤��CaCl2��������Na2CO3����ȫ��Ӧ���ķ�����: ���ã����ϲ���Һ�еμ�CaCl2��Һ�����û�а�ɫ�������ɣ�֤��CaCl2�������۹��ˡ�����X�������������������Ϊ10��0g������X��������ϴ�ӣ���ֹ������ճ��NaCl�����ȱ�ٴ˲��������̼��������ƫ����������ƫ�ߣ��ܷ�����Ӧ�ķ���ʽΪ��CaCl2+Na2CO3==CaCO3��+2NaCl������������Ϊ10��0g����̼��Ƶ����������ݷ���ʽ��Na2CO3��̼��Ƶ�������ϵ���������Na2CO3����������һ�������Ʒ��̼���Ƶ���������
�⣺����Ʒ��̼���Ƶ�����Ϊx
Na2CO3+CaCl2 CaCO3��+2Na Cl
CaCO3��+2Na Cl
106 100
x 10��0g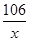 =
=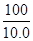
x=10��6g
��̼���Ƶ�����������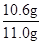 ��100%=96��4%
��100%=96��4%
���㣺ʵ�����ݵķ��������������ݻ�ѧ����ʽ���еļ���

������ɶ�ʵ������ȡ������̼��ʵ��̽����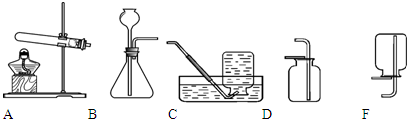
��1��ѡ��ҩƷ���±��Ƕ�����ҩƷ����ʵ��ļ�¼��
| ��� | ҩƷ | ʵ������ |
| �� | ��״ʯ��ʯ��ϡ���� | ���������������� |
| �� | ʯ��ʯ��ĩ��ϡ����[��] | �����������ʺܿ� |
| �� | ��״ʯ��ʯ��ϡ���� | �����������ʻ�������ֹͣ |
| �� | ̼���Ʒ�ĩ��ϡ���� | �����������ʺܿ� |
����ȡ���ռ��ĽǶȷ�����һ��ѡ��� ��ҩƷ������ĸ��ţ���ͬ��������ҩƷ������Ӧ�Ļ�ѧ����ʽΪ ��
��2��ѡ��װ�á�ѡ�� ����װ�á�
��3����ȡ���塣ѡ�� װ���ռ�������̼�������������� ��
��4��������顣�����ɵ�����ͨ����ɫʯ����Һ�У���Һ��죬ȷ���������Ƕ�����̼�����ּ��鷽���Ƿ���ȷ������ȷ��˵�����ɣ�������ȷ��д����ȷ�ļ��鷽���� ��
6��5���ǡ����绷���ա���2013���й�����Ϊ��ͬ�������ܶ�����
��1����ʯȼ���Dz���������Դ������ú�� ���� ����Ȼ���ȡ�
��2�����в����ڿ�����Ⱦ����� ���� ��
| A���������� | B���������� | C��PM2.5 | D������ |
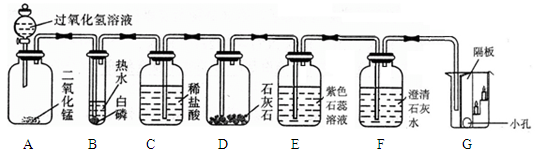
����Һ©��������A�г��ִ������ݣ�B�а���ȼ�գ�C��Һ���½���ϡ��������D�С�E����ɫʯ����Һ��ɺ�ɫ��F������ʯ��ˮ����ǡ�
��A�з�����Ӧ�Ļ�ѧ����ʽΪ ���� ��
��B�а����ܹ�ȼ�յ�ԭ���� ���� ��
��G�ձ��� ���� ����ϲ㡱���²㡱����������Ϩ��˵��������̼���е����������� ���� �����еĻ�ѧ������ ���� ��
��E��ʹ��ɫʯ���ɺ�ɫ���������� ����д��F�������仯�Ļ�ѧ����ʽ�� ����
��ȥ���������е��������ʣ�������ȷ���ǣ�������
| ѡ�� | ���ʣ����ʣ� | ��ȥ���ʵķ��� |
| A | ������������ | �������ȵ�ͭ�� |
| B | �Ȼ��أ��������̣� | ��ˮ�ܽ⣬���ˣ�ϴ�Ӹ��� |
| C | ��ʯ�ң�̼��ƣ� | �������� |
| D | ����������Һ��̼���ƣ� | ���������Ȼ�����Һ������ |