��Ŀ����
(8��)��ͷ�������ĺ������У������Ƿḻ�Ļ�ѧ��Դ���⣬ͨ����ɹ��ˮ���Եõ����н϶����ʵĴ��Ρ�
(1)�������γ����ᴿ��ʵ��ʱ��Ҫ��������ͼ��ʾ��ʵ�������
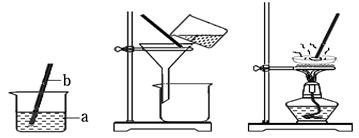
�ٲ���B�в�������������
�ڲ���C�п��� ʱ��ֹͣ���ȡ�
(2)����ƽ����һ�����ĺ��Ȼ�þ�������ƺ��Ȼ��ƵĴ��Σ���������������̽��г����ᴿ����һ���õ��ϴ������Ȼ��ƹ��塣
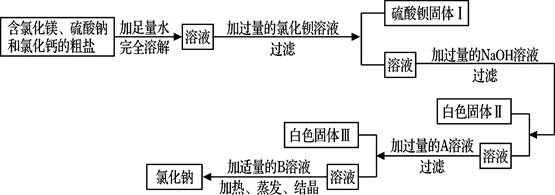
��������ͼ�ش��������⣺
�١����������A��Һ��������A�Լ��� ��
��д����Ӧ�����м����Ȼ�����Һ�����������ᱵ�Ļ�ѧ��Ӧ����ʽ ��
�۰�ɫ�����ijɷ���Mg(OH)2����ɫ�����ijɷ��� �� ��
�ܼ�������B��Һ��B��Һ�� ��
(1)�������γ����ᴿ��ʵ��ʱ��Ҫ��������ͼ��ʾ��ʵ�������

�ٲ���B�в�������������
�ڲ���C�п��� ʱ��ֹͣ���ȡ�
(2)����ƽ����һ�����ĺ��Ȼ�þ�������ƺ��Ȼ��ƵĴ��Σ���������������̽��г����ᴿ����һ���õ��ϴ������Ȼ��ƹ��塣

��������ͼ�ش��������⣺
�١����������A��Һ��������A�Լ��� ��
��д����Ӧ�����м����Ȼ�����Һ�����������ᱵ�Ļ�ѧ��Ӧ����ʽ ��
�۰�ɫ�����ijɷ���Mg(OH)2����ɫ�����ijɷ��� �� ��
�ܼ�������B��Һ��B��Һ�� ��
(1)�������϶���� (2)��Na2CO3 ��BaCl2+ Na2SO4=BaSO4��+2NaCl(2��)
��CaCO3��BaCO3 ��ϡ����
��CaCO3��BaCO3 ��ϡ����
��1���ٲ������������������������������п����������г��ֽ϶����ʱ��ֹͣ���ȣ������Ȱ�ˮ�����ɣ�
��2����ȥ�����е��Ȼ��ƣ��Ȼ�þ�������ƣ�����������̼���ƣ���ȥþ�������������ƣ���ȥ��������Ȼ�����Ҫע������Լ���˳��������������Լ�Ҫ��ǰ�����Ĺ����Լ�Ҳ��ȥ��
�١����������A��Һ����Ŀ���dz�ȥ�Ȼ��ƺ��Ȼ���������A�Լ��� Na2CO3��
�ڰ�ɫ�����ijɷ��Ǽ����������ƺ����ɵģ����� Mg��OH��2��������ɫ�����ijɷ����Ȼ��ƺ��Ȼ��������̼�������ɵģ��ʳ����� CaCO3 BaCO3�������ʣ�
�ۼ�������B��Һʱ��ԭ��Һ�к���̼���ƺ������������ʣ��ʼ����B��Һ��ϡ���ᣬĿ���dz�ȥ������̼���ƺ��������ƣ�
��2����ȥ�����е��Ȼ��ƣ��Ȼ�þ�������ƣ�����������̼���ƣ���ȥþ�������������ƣ���ȥ��������Ȼ�����Ҫע������Լ���˳��������������Լ�Ҫ��ǰ�����Ĺ����Լ�Ҳ��ȥ��
�١����������A��Һ����Ŀ���dz�ȥ�Ȼ��ƺ��Ȼ���������A�Լ��� Na2CO3��
�ڰ�ɫ�����ijɷ��Ǽ����������ƺ����ɵģ����� Mg��OH��2��������ɫ�����ijɷ����Ȼ��ƺ��Ȼ��������̼�������ɵģ��ʳ����� CaCO3 BaCO3�������ʣ�
�ۼ�������B��Һʱ��ԭ��Һ�к���̼���ƺ������������ʣ��ʼ����B��Һ��ϡ���ᣬĿ���dz�ȥ������̼���ƺ��������ƣ�

��ϰ��ϵ�д�
�����Ŀ

