��Ŀ����
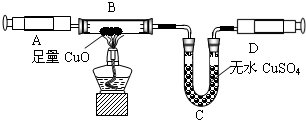
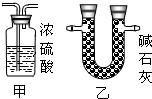
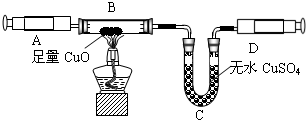
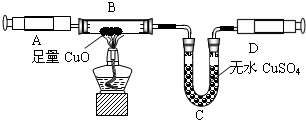
ijͬѧ�ڲ�������ʱ��֪�����۴�����Ʒ�������Ȼ������ʣ�| ���Ͽ��� �����Ƽ��ԭ�����Ա�ʾΪ�� NaCl+H2O+NH3+CO2 =NaHCO3��+NH4Cl 2NaHCO3?Na2CO3+CO2 ��+H2O��ͬѧ�������ͼ�е�װ�ý���ʵ�飬�ⶨ���۴�����Ʒ��̼���Ƶ�������������ˮ������Ӱ����Բ��ƣ��г�װ������ȥ��  ��Ҫʵ�鲽�����£� �ٰ�ͼ��װ�����������װ�õ������ԣ� �ڳ���5.5g���۴�����Ʒ������ƿ�У�����������ˮ�ܽ⣬�õ�������Һ�� �۴ӷ�Һ©������ϡ���ᣬֱ�����ٲ�������ʱΪֹ�� �ܻ�������һ������N2�� �ݳ���Bƿ����Һ����������������2.2g�� ��ش��������⣺ ��1��д��A�з�����Ӧ�Ļ�ѧ����ʽ ��2������һ������N2��Ŀ���� ��3���������Һ©���е�ϡ���ỻ��Ũ���ᣬ���ԵĽ������ƫ�ߡ�ƫ�ͻ䣩 ��4������Ʒ��̼���Ƶ���������Ϊ ��5������������ʵ�鷴Ӧԭ����ͬ��ʵ�����ⶨ������Ʒ��̼���Ƶ��������������û�ѧ����ʽ��ʾʵ��ԭ�� ��������1������װ��A������ҩƷ������ҩƷ��ķ�Ӧ���ɣ�д����Ӧ�Ļ�ѧ����ʽ�� ��2������ʵ��IJ�������������ʵ��Ĺ��̣�����ʵ�������ͨ�뵪���������ü�װ��C�г���ʯ��ˮ��ʵ������е����ã� ��3���Ա�ϡ������Ũ���������ϵIJ��죬������ʹ��Ũ������ʵ������ɵ�Ӱ�죻 ��4������ʵ�������ɶ�����̼���������ɷ�Ӧ�Ļ�ѧ����ʽ�������Ʒ��̼���Ƶ��������������Ʒ��̼���Ƶ����������� ��5������̼���Ƶ����ʼ��仯���ɣ���ʹ̼�������Ȼ��Ƶ��γɳ����ȣ��������ʵ�����̼�������������IJⶨ�� ����⣺ ��1����Ʒ���Ȼ��Ʋ������ᷴӦ��̼���������ᷴӦ���������ơ�ˮ�Ͷ�����̼����ѧ����ʽΪNa2CO3+H2SO4�TNa2SO4+H2O+CO2���� ��2��Ϊʹ�����ڷ�Ӧ���������ɵĶ�����̼ȫ���������������գ�ͨ�벻�����������Ʒ�����Ӧ�ĵ����ų���Ӧװ���ڵĶ�����̼��װ��C��ʢ�ų���ʯ��ˮ���������̼�����Ƿ�ȫ���������������գ� ��3��ϡ����ӷ���Ũ������к�ǿ�Ļӷ��ԣ��ӷ�����HCl���屻����������Һ���պ��²�ö�����̼������ƫ�ߣ� ��4��������������2.2g��˵����Ӧ�ų�2.2g������̼���跴Ӧ����̼���Ƶ�����Ϊx Na2CO3+H2SO4�TNa2SO4+H2O+CO2�� 106 44 x 2.2g
����Ʒ��̼���Ƶ���������=
��5��̼���������Ȼ��Ƶȷ�Ӧ����̼��Ƴ�������˿�ѡ���Ȼ�����Һ��̼���Ʒ�Ӧ��ͨ���ⶨ���ɳ���������Ȼ��Ƶ������ⶨ��Ʒ��̼���Ƶ����������õ���Ʒ��̼���Ƶ�����������̼������Ȼ��Ʒ�Ӧ�Ļ�ѧ����ʽΪNa2CO3+CaCl2�TCaCO3��+2NaCl�� �ʴ�Ϊ�� ��1��Na2CO3+H2SO4�TNa2SO4+H2O+CO2���� ��2���ų�װ��A�������в���CO2��ʹ��ȫ����װ��B��NaOH��Һ���գ�����CO2�Ƿ�Bƿ�е�NaOH��Һ��ȫ���գ� ��3��ƫ�ߣ� ��4��96.4%�� ��5��Na2CO3+CaCl2�TCaCO3��+2NaCl������BaCl2��Ba��OH��2��Ӧ���� ������������Ҫ����ѧ�����û�ѧ����ʽ����������������ʽ�ۺϷ����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ�������� 
��ϰ��ϵ�д�
�����Ŀ
ij��ȤС��ͬѧΪ֤��NaOH��Һ��ϡ���ᷢ�����кͷ�Ӧ���Ӳ�ͬ�Ƕ����������ʵ�鷽����������ʵ�飮
����һ������pH��ֽ�ⶨNaOH��Һ��pH���ٵμ����ᣬ����������Һ��ͬʱ�ⶨ�����Һ��pH�������õ�pH��С��С��7����֤��NaOH��Һ��ϡ���ᷢ���˻�ѧ��Ӧ�� ��1����pH��ֽ�ⶨ��Һ��pHʱ����ȷ�IJ����ǣ� ��2������ǿ������õ�pHС��7�������ɣ� ������������NaOH��Һ�еμӼ��η�̪��Һ����Һ�Ժ�ɫ��Ȼ���ٵμ����ᣬ�ɹ۲쵽��ɫ����ʧ����֤��NaOH��Һ��ϡ���ᷢ���˻�ѧ��Ӧ�� ����ͬѧ����NaOH��Һ�еμӷ�̪��Һʱ��������һ��������������������Һ�е����̪��Һ����Һ����˺�ɫ������һ�����ɫ����ʧ�ˣ���С����������������ԭ���������²��룺�ٿ����Ƿ�̪��Һ������е�������Ӧ��ʹ��ɫ��ʧ���ڿ���������������Һ������еĶ�����̼��Ӧ��ʹ��ɫ��ʧ�� ��1��Ϊ��֤����٣�����ͬѧ��������ʵ�飺�����Ƶ�����������Һ���ȣ�����Һ���Ϸ���һЩֲ���ͣ�Ȼ������ȴ�����Һ�е����̪��Һ��ʵ���С����ȡ��͡�����ֲ���͡�Ŀ���� ��2��Ϊ��֤����ڣ�����ͬѧ��������ʵ�飺ȡ��һ������Na2CO3��Һ�������е����̪��Һ��������ҺҲ���ֺ�ɫ���ɴ˿ɵó�����������ۣ�����1��˵��Na2CO3��Һ�� ��3����С��ͬѧͨ���������ϵ�֪��������������ҺŨ�Ⱥܴ�ʱ���ͻ���������������������ʵ��֤���÷�����ȡ�õ�NaOH��ҺŨ�ȹ���ʵ�鷽�� ����������ѧ��Ӧ��ͨ�������������ı仯���ɽ�����Ӧǰ����¶ȱ仯���жϷ�Ӧ�ķ��������NaOH��Һ��ϡ������ǰ���¶��б仯����֤�������˻�ѧ��Ӧ�� ����ͬѧ����ͬŨ�ȵ������NaOH��Һ��10mL��ϣ����� �ȼƲⶨ�����»��ǰ���¶ȵı仯������¼��ÿ�λ��ǰ���¶ȵ�����ֵ��t���������
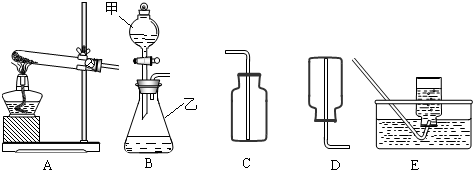
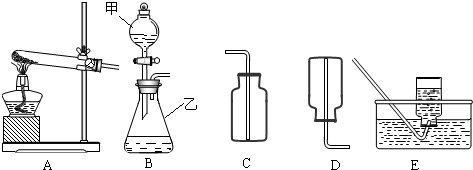
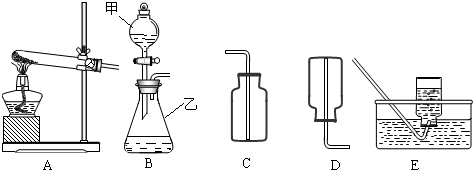
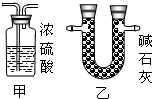
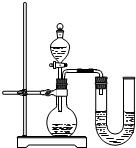 ��1������x= ��2��ijͬѧ��ûʹ���¶ȼƵ�����£�ͨ����ͼ��ʾװ�������ʵ�飮���ͬѧ���� ��3������ʵ���е�ϸ�ں������������ʵ���У�ϡ��������ý�ͷ�ι���εμӣ���������Ŀ���� ��4��Ϊ�˽�һ���о�ʵ���г��ֵ����⣬ȡ��13.3g�������ƹ�����Ʒ��������ˮ�����Һ�������м���200g10%��ϡ���ᣬʹ���ַ�Ӧ�����ɶ�����̼2.2g���� ��1����Ʒ���������Ƶ������� ��2�����������Ʒ�Ӧ������������� ��3����ͼ�л������������ʾ������̼�������������ʾ����������Ĺ�ϵͼ��  ����֪Na2CO3+2HCl�T2NaCl+H2O+CO2���� |