��Ŀ����
����Ŀ����Դ��������������������ᷢչ������أ�
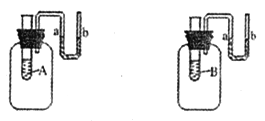
��1��Ϊ������Ⱦ����������ʣ��ɽ�úת��Ϊ��ȼ�����壨ͼΪ��ʾ��ͼ����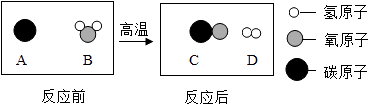
�÷�Ӧ�Ļ�ѧ����ʽ����
��2����ͪ��C3H6O����һ�ֺܺõ��л��ܼ�������ȼ���ӷ�����д����ͪ�ڿ�����ȼ�յĻ�ѧ����ʽ����
��3��һ�������£�һ����̼��������Ӧ����������ֻ��һ�֣��ͷ��ϡ���ɫ��ѧ���ġ����ŷš����������������������������ĸ����
A.�״���CH4O��
B.���ᣨCH2O2��
C.��ȩ��C2H4O��
���𰸡�
��1��C+H2O ![]() CO+H2
CO+H2
��2��C3H6O+4O2 ![]() 3CO2+3H2O
3CO2+3H2O
��3��A
���������⣺��1���ɷ�Ӧ����ʾ��ͼ��֪�÷�Ӧ��̼��ˮ�ڸ��������·�Ӧ������һ����̼����������Ӧ�Ļ�ѧ����ʽ�ǣ�C+H2O ![]() CO+H2����2����ͪ�ڿ�����ȼ�������˶�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��C3H6O+4O2
CO+H2����2����ͪ�ڿ�����ȼ�������˶�����̼��ˮ����Ӧ�Ļ�ѧ����ʽΪ��C3H6O+4O2 ![]() 3CO2+3H2O����3��A�����ݻ�ѧʽCH4O����֪��������C��OԪ�ص�ԭ�Ӹ�����Ϊ1��1�������������л�����ص㣬����ȷ��
3CO2+3H2O����3��A�����ݻ�ѧʽCH4O����֪��������C��OԪ�ص�ԭ�Ӹ�����Ϊ1��1�������������л�����ص㣬����ȷ��
B�����ݻ�ѧʽCH2O2����֪��������C��OԪ�ص�ԭ�Ӹ�����Ϊ1��2���������������л�����ص㣬�ʴ���
C�����ݻ�ѧʽC2H4O����֪��������C��OԪ�ص�ԭ�Ӹ�����Ϊ2��1���������������л�����ص㣬�ʴ���
�ʴ�Ϊ����1��C+H2O ![]() CO+H2����2��C3H6O+4O2
CO+H2����2��C3H6O+4O2 ![]() 3CO2+3H2O����3��A��
3CO2+3H2O����3��A��
�����㾫����������ɫ��ѧ����д��ѧ����ʽ�����ֱ���ʽ�����뷽��ʽ����Ŀ�����жϼ��ɵõ��𰸣���Ҫ��֪��ɫ��ѧ-----�����Ѻû�ѧ(���Ϸ�Ӧ������ɫ��ѧ��Ӧ)�����ص�P6��ԭ�ϡ����������ŷš���Ʒ���ں��ģ����û�ѧԭ����Դͷ������Ⱦ��ע�⣺a����ƽ b������ c�����ţ�