��Ŀ����
ijУͬѧ���г��ϵ�ʳƷ���ɼ�����������̽����ʵ��һ����С���ͬѧ�ռ���һ����Ҫ�ɷ���С�մ�̼�����ƣ������ɼ�
��1��ȡ��Ʒ����������ˮ�У������Һ��pH����7��
��2����С�մ���Ʒ�еμ����ᣬ�д������ݲ�����������������Ƕ�����̼���÷�Ӧ�Ļ�ѧ����ʽΪ______��
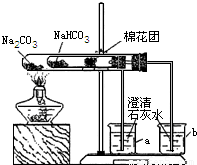
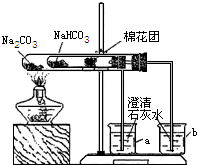
��3��ͬѧ��ͨ���������ϣ�����ʦ��ָ���²�������ͼ��ʾװ����С�մ�ʹ������˶Ա�ʵ�飮ʵ����С�Թ���մ����ˮ����ͭ��ĩ����������ɫ��a�ձ���ʯ��ˮ����������
b�ձ���ʯ��ˮ���ְ�ɫ���ǣ��Իش𣺢���ʵ�����ʱ��������Ҫע��������ǣ�______
����ʵ�飨3�����Եó��Ľ�����______������ũ������á����桱��̼���ƣ�����ͷʱ�������Ƚ���۷��ͣ�����һЩ�л��ᣩ��ԭ����______��
ʵ�������С���ͬѧ�ռ���һ����Ϊ�����ۡ������ɼ���Ϊ̽������ɣ�����ʵ�飺
��1�������ۡ�Ϊһ�ְ�ɫ���壬������ˮ��
��2��ȡ������Ʒ��ϡ�����Ϻ�����˿���ʹʯ��ˮ����ǵ����壻
��3��ȡ������Ʒ���Ⱥ������д̼�����ζ�����壻
��4��������Ʒ������������Һ��Ϻ��ȣ�������ʹʪ��ĺ�ɫʯ����ֽ�����ij�����
������֪�������ۡ�����Ҫ�ɷ��dz��л�ѧ�α���һ�ֳ������Σ�
������Ϊ������______�������Ⱥ�Ӧ�ķ���ʽΪ______ NH3��+H2O+CO2��
���𰸡�������̼���ƺ�̼��������ʳƷ��ҵ���õ��Լ����������������ȶ��Բ�ͬ�����Ƕ��Լ��ԣ��������ᷴӦ���ɶ�����̼���壬̼������к��е�笠�������������ʻ�ϻ������д̼�����ζ�İ��������е�̼��������������������ɶ�����̼���壮
����⣺ʵ��һ����2��̼�������������ᷴӦ�����Ȼ��ơ�������̼��ˮ�����Բ�ͬ��Ϊ��NaHCO3+HCl�TNaCl+H2O+CO2����
��3���ٵ�������Һ���У��������Թܽ�����Ϊ��ֹҺ�嵹��ʹ�Թ�ը�ѣ���Ҫ�Ƚ������Ƴ�����Ϩ��ƾ��ƣ����Ա����Ϊ���Ƚ��ձ�a��b�еĵ������Ƴ�����Ϩ��ƾ��ƣ�
�ڴ��Թ��ڷŵ���̼���ƣ�a�ձ���ʯ��ˮ����������˵��̼�������Ȳ��ֽ⣬С�Թ��ڷŵ���̼�����ƣ����Ⱥ���ˮ����ͭ������˵����ˮ���ɣ�b�ձ���ʯ��ˮ���ְ�ɫ���ǣ�˵���ж�����̼���ɣ��Ӷ�˵��̼�����������ֽ⣬���ŷ����ܲ����ᣬ�ü�������ͷ������ʹ���ŷ��Ͳ���������̼���Ʒ�Ӧ���ɶ�����̼�����Ա����Ϊ��̼�������Ȳ��ֽ⣬̼���������ȷֽ��ˮ��CO2�����ʣ�ʹ�������ᷴӦ����CO2���壨̼�������Ȳ��ֽ⣩��
ʵ������ٸ����������ᷴӦ���ɶ�����̼���壬�������ֽ⣬˵���������к�̼��������ӣ���������ʻ���ܲ�����ʹʪ��ĺ�ɫʯ����ֽ�����ij�����˵���������к���笠����ӣ��ʸ�����Ϊ̼����泥����Ա����Ϊ��NH4HCO3��
NH4HCO3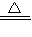 NH3��+H2O+CO2����
NH3��+H2O+CO2����
�ڡ����ۡ������ɼ�����ըʳƷ������д̼�����ζ�İ���������Ҫ����һ��ʱ�䣬ʹ�����İ����ӷ��������Ա����Ϊ��ʹ��ը�����İ����ӷ���
��̼�������̼������ĺ�����̼��������̼������ĺ����ߣ�����������ʵ�������ͬ��̼������������������̼�����ƶ࣬���Ա����Ϊ����������̼������������������̼�����ƶ࣮
ʵ��������1��С�մ����ۡ��ж�����̼��������ӣ��������Ƶ����ʣ��������ᷴӦ���ɶ�����̼�������ȶ��������ֽ⣬���Ա����Ϊ��CO2�����ȷֽ��������̼�����ᷴӦ����������̼��
��2���ý�������ۼ���ˮ��ȡ�ϲ�Һ����pH��ֽ��ã�pH=7��˵����Һ�����ԣ�Ӧ�������ȷֽ�����˶�����̼�����Ա����Ϊ�����ȷֽ���Ķ�����̼��
ʵ���ģ���1�������ɼ��к�̼�����ƺ�ijЩ�������ʣ�����ʹ�ú�����ɶ�����̼������̼�����Ƶ����ʣ���֪����������̼��;�����������ᷴӦ��Ҳ���������ȷֽ⣬���Ա����Ϊ��CO2�����ȷֽ��������̼�����ᷴӦ����������̼��
��2�����ۣ�
��Ҫ�ⶨ������̼�����������뱣֤װ�ò�©�������Խ���ʵ��ǰҪ���װ�õ������ԣ�ʹ���ɼ�����������̼�ɼ���ϡ���ᣬ������������ʹ������̼�л����Ȼ������壬���Ա����Ϊ��װ�õ������ԣ�ϡ���
����װ��ʢ��Ũ���������ˮ�ԣ��ʿ��������������̼����װ��ʢ��������������Һ���������տ����еĶ�����̼����ֹ�ⶨ�����ݲ�ȷ�����Ա����Ϊ�����ն�����̼�л��е�ˮ���������չ�������еĶ�����̼��
�۽⣺�������꣬���ɶ�����̼������Ϊ22g����̼�����Ƶ�����Ϊx����
2NaHCO3+H2SO4=Na2SO4+2CO2��+2H2O
168 88
x 22g
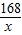 =
=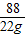
x=42g
��NaHCO3%=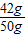 ×100%
×100%
=84%
��������̼�����Ƶ���������Ϊ84%��
�����������ۺϿ�����̼���ơ�̼�����ƺ�̼����淋�֪ʶ���ش�����ʱ���������ݿα����е�֪ʶ�����Ӧ�ã�����ͬѧ����ƽʱ��ѧϰ��Ҫ��ǿ֪ʶ��������Ӧ�ã�
����⣺ʵ��һ����2��̼�������������ᷴӦ�����Ȼ��ơ�������̼��ˮ�����Բ�ͬ��Ϊ��NaHCO3+HCl�TNaCl+H2O+CO2����
��3���ٵ�������Һ���У��������Թܽ�����Ϊ��ֹҺ�嵹��ʹ�Թ�ը�ѣ���Ҫ�Ƚ������Ƴ�����Ϩ��ƾ��ƣ����Ա����Ϊ���Ƚ��ձ�a��b�еĵ������Ƴ�����Ϩ��ƾ��ƣ�
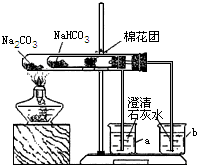
�ڴ��Թ��ڷŵ���̼���ƣ�a�ձ���ʯ��ˮ����������˵��̼�������Ȳ��ֽ⣬С�Թ��ڷŵ���̼�����ƣ����Ⱥ���ˮ����ͭ������˵����ˮ���ɣ�b�ձ���ʯ��ˮ���ְ�ɫ���ǣ�˵���ж�����̼���ɣ��Ӷ�˵��̼�����������ֽ⣬���ŷ����ܲ����ᣬ�ü�������ͷ������ʹ���ŷ��Ͳ���������̼���Ʒ�Ӧ���ɶ�����̼�����Ա����Ϊ��̼�������Ȳ��ֽ⣬̼���������ȷֽ��ˮ��CO2�����ʣ�ʹ�������ᷴӦ����CO2���壨̼�������Ȳ��ֽ⣩��
ʵ������ٸ����������ᷴӦ���ɶ�����̼���壬�������ֽ⣬˵���������к�̼��������ӣ���������ʻ���ܲ�����ʹʪ��ĺ�ɫʯ����ֽ�����ij�����˵���������к���笠����ӣ��ʸ�����Ϊ̼����泥����Ա����Ϊ��NH4HCO3��
NH4HCO3
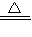 NH3��+H2O+CO2����
NH3��+H2O+CO2�����ڡ����ۡ������ɼ�����ըʳƷ������д̼�����ζ�İ���������Ҫ����һ��ʱ�䣬ʹ�����İ����ӷ��������Ա����Ϊ��ʹ��ը�����İ����ӷ���
��̼�������̼������ĺ�����̼��������̼������ĺ����ߣ�����������ʵ�������ͬ��̼������������������̼�����ƶ࣬���Ա����Ϊ����������̼������������������̼�����ƶ࣮
ʵ��������1��С�մ����ۡ��ж�����̼��������ӣ��������Ƶ����ʣ��������ᷴӦ���ɶ�����̼�������ȶ��������ֽ⣬���Ա����Ϊ��CO2�����ȷֽ��������̼�����ᷴӦ����������̼��
��2���ý�������ۼ���ˮ��ȡ�ϲ�Һ����pH��ֽ��ã�pH=7��˵����Һ�����ԣ�Ӧ�������ȷֽ�����˶�����̼�����Ա����Ϊ�����ȷֽ���Ķ�����̼��
ʵ���ģ���1�������ɼ��к�̼�����ƺ�ijЩ�������ʣ�����ʹ�ú�����ɶ�����̼������̼�����Ƶ����ʣ���֪����������̼��;�����������ᷴӦ��Ҳ���������ȷֽ⣬���Ա����Ϊ��CO2�����ȷֽ��������̼�����ᷴӦ����������̼��
��2�����ۣ�
��Ҫ�ⶨ������̼�����������뱣֤װ�ò�©�������Խ���ʵ��ǰҪ���װ�õ������ԣ�ʹ���ɼ�����������̼�ɼ���ϡ���ᣬ������������ʹ������̼�л����Ȼ������壬���Ա����Ϊ��װ�õ������ԣ�ϡ���
����װ��ʢ��Ũ���������ˮ�ԣ��ʿ��������������̼����װ��ʢ��������������Һ���������տ����еĶ�����̼����ֹ�ⶨ�����ݲ�ȷ�����Ա����Ϊ�����ն�����̼�л��е�ˮ���������չ�������еĶ�����̼��
�۽⣺�������꣬���ɶ�����̼������Ϊ22g����̼�����Ƶ�����Ϊx����
2NaHCO3+H2SO4=Na2SO4+2CO2��+2H2O
168 88
x 22g
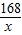 =
=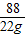
x=42g
��NaHCO3%=
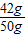 ×100%
×100%=84%
��������̼�����Ƶ���������Ϊ84%��
�����������ۺϿ�����̼���ơ�̼�����ƺ�̼����淋�֪ʶ���ش�����ʱ���������ݿα����е�֪ʶ�����Ӧ�ã�����ͬѧ����ƽʱ��ѧϰ��Ҫ��ǿ֪ʶ��������Ӧ�ã�

��ϰ��ϵ�д�
 ��ĩ1�����ʽ���������ϵ�д�
��ĩ1�����ʽ���������ϵ�д�
�����Ŀ
 ijУͬѧ���г��ϵ�ʳƷ���ɼ�����������̽����
ijУͬѧ���г��ϵ�ʳƷ���ɼ�����������̽����ʵ��һ����С���ͬѧ�ռ���һ����Ҫ�ɷ���С�մ�̼�����ƣ������ɼ�
��1��ȡ��Ʒ����������ˮ�У������Һ��pH����7��
��2����С�մ���Ʒ�еμ����ᣬ�д������ݲ�����������������Ƕ�����̼���÷�Ӧ�Ļ�ѧ����ʽΪ
��3��ͬѧ��ͨ���������ϣ�����ʦ��ָ���²�������ͼ��ʾװ����С�մ�ʹ������˶Ա�ʵ�飮ʵ����С�Թ���մ����ˮ����ͭ��ĩ����������ɫ��a�ձ���ʯ��ˮ����������
b�ձ���ʯ��ˮ���ְ�ɫ���ǣ��Իش𣺢���ʵ�����ʱ��������Ҫע��������ǣ�
����ʵ�飨3�����Եó��Ľ�����
ʵ�������С���ͬѧ�ռ���һ����Ϊ�����ۡ������ɼ���Ϊ̽������ɣ�����ʵ�飺
��1�������ۡ�Ϊһ�ְ�ɫ���壬������ˮ��
��2��ȡ������Ʒ��ϡ�����Ϻ�����˿���ʹʯ��ˮ����ǵ����壻
��3��ȡ������Ʒ���Ⱥ������д̼�����ζ�����壻
��4��������Ʒ������������Һ��Ϻ��ȣ�������ʹʪ��ĺ�ɫʯ����ֽ�����ij�����
������֪�������ۡ�����Ҫ�ɷ��dz��л�ѧ�α���һ�ֳ������Σ�
������������
���á����ۡ������ɼ�����ըʳƷ���ú������ۺ�ʳ�õ�ԭ�������
��ijЩС�̷�ϲ��ѡ�á����ۡ����桰С�մ������ɼ���ԭ�������
ʵ��������С��ͬѧ̽��С�մ����ۡ�������ʳƷ�����в������ݵ�ԭ��
��1���������ɼ������������ж�����
��2��С���ͬѧ��������ۼ���ˮ��ȡ�ϲ�Һ����pH��ֽ��ã�pH=7���²���������ԭ����
ʵ���ģ���С���ͬѧ��ij���з���һ�ָ������ɼ�--���ͷۣ���ɷּ�����
| �ɷ� | �������� |
| ̼������ | ̼������ |
| �������� | �����ᡢ��ʯ��� |
| ���ɼ� | ���� |
| �����ɷ� | ���ۡ�֬����� |
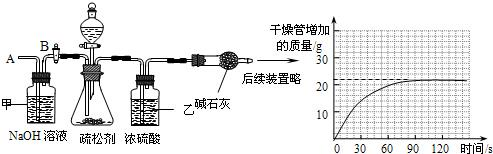
��2��Ϊ�ⶨ����̼�����Ƶ�����������С���ͬѧ���������ʵ�飬����Ҫʵ�鲽�����£�
��ͼ��װ��������50.0g����������ƿ�У���������ij����Һ��
����һ��ʱ���ڸ�������������ӵ����������������ߣ���
����ƿ�в��ٲ�������ʱ������B���ӵ���A����������һ�����Ŀ�����
�����ٴγ�����������������ӵ�������
�����ظ���͢��IJ�����ֱ��������������������䣮
���ۣ�
�ٲ��������Ʒǰ��Ӧ���
��װ���ҵ�������
���������й����ݼ���������̼�����Ƶ�������������д��������̣���С��4�֣�
 ijУͬѧ���г��ϵ�ʳƷ���ɼ�����������̽����
ijУͬѧ���г��ϵ�ʳƷ���ɼ�����������̽����
ʵ��һ����С���ͬѧ�ռ���һ����Ҫ�ɷ���С�մ�̼�����ƣ������ɼ�
��1��ȡ��Ʒ����������ˮ�У������Һ��pH����7��
��2����С�մ���Ʒ�еμ����ᣬ�д������ݲ�����������������Ƕ�����̼���÷�Ӧ�Ļ�ѧ����ʽΪ________��
��3��ͬѧ��ͨ���������ϣ�����ʦ��ָ���²�������ͼ��ʾװ����С�մ�ʹ������˶Ա�ʵ�飮ʵ����С�Թ���մ����ˮ����ͭ��ĩ����������ɫ��a�ձ���ʯ��ˮ����������
b�ձ���ʯ��ˮ���ְ�ɫ���ǣ��Իش𣺢���ʵ�����ʱ��������Ҫע��������ǣ�________
����ʵ�飨3�����Եó��Ľ�����________������ũ������á����桱��̼���ƣ�����ͷʱ�������Ƚ���۷��ͣ�����һЩ�л��ᣩ��ԭ����________��
ʵ�������С���ͬѧ�ռ���һ����Ϊ�����ۡ������ɼ���Ϊ̽������ɣ�����ʵ�飺
��1�������ۡ�Ϊһ�ְ�ɫ���壬������ˮ��
��2��ȡ������Ʒ��ϡ�����Ϻ�����˿���ʹʯ��ˮ����ǵ����壻
��3��ȡ������Ʒ���Ⱥ������д̼�����ζ�����壻
��4��������Ʒ������������Һ��Ϻ��ȣ�������ʹʪ��ĺ�ɫʯ����ֽ�����ij�����
������֪�������ۡ�����Ҫ�ɷ��dz��л�ѧ�α���һ�ֳ������Σ�
������Ϊ������________�������Ⱥ�Ӧ�ķ���ʽΪ________
���á����ۡ������ɼ�����ըʳƷ���ú������ۺ�ʳ�õ�ԭ�������________��
��ijЩС�̷�ϲ��ѡ�á����ۡ����桰С�մ������ɼ���ԭ�������________��
ʵ��������С��ͬѧ̽��С�մ����ۡ�������ʳƷ�����в������ݵ�ԭ��
��1���������ɼ������������ж�����________���Ʋ�������������ԭ����________��________��
��2��С���ͬѧ��������ۼ���ˮ��ȡ�ϲ�Һ����pH��ֽ��ã�pH=7���²���������ԭ����________��
ʵ���ģ���С���ͬѧ��ij���з���һ�ָ������ɼ�--���ͷۣ���ɷּ�����
| �ɷ� | �������� |
| ̼������ | ̼������ |
| �������� | �����ᡢ��ʯ��� |
| ���ɼ� | ���� |
| �����ɷ� | ���ۡ�֬����� |
��2��Ϊ�ⶨ����̼�����Ƶ�����������С���ͬѧ���������ʵ�飬����Ҫʵ�鲽�����£�
��ͼ��װ��������50.0g����������ƿ�У���������ij����Һ��
����һ��ʱ���ڸ�������������ӵ����������������ߣ���
����ƿ�в��ٲ�������ʱ������B���ӵ���A����������һ�����Ŀ�����
�����ٴγ�����������������ӵ�������
�����ظ���͢��IJ�����ֱ��������������������䣮

���ۣ�
�ٲ��������Ʒǰ��Ӧ���________�����ӵ�����Һ��________��
��װ���ҵ�������________���ӵ���A����������һ�����Ŀ���ʱ��װ�ü�������________��
���������й����ݼ���������̼�����Ƶ�������������д��������̣���С��4�֣�