��Ŀ����
�����С��һ��̽������Һ�ijɷ֣����ⶨ������������
��һ�������벢��֤����Һ��������ʲô��
���룺���ݱ�ǩ��֪����ƿ��Һ������HCl��KCl��NaCl��
���������ӵ�����Σ�
��֤��
| ���� | ���� | ���� |
| ȡ�����μ���ɫʯ�� | ��Һ���______ɫ | ��ƿ��Һ������ |
ʵ��ԭ������1������֪Ũ�ȵ�����������Һ�����ᷴӦ����Ӧ�Ļ�ѧ����ʽΪ��
NaOH+HCl=NaCl+H20
��2�����������������ǡ����ȫ��Ӧʱ��������һ������������Һ����Һ�ͳʼ��ԣ�����ʹ��ɫ��̪��ɺ�ɫ������һ������������Һ�����ԼΪ0.05mL���Բⶨ�����Ӱ���С���ɺ��Բ��ƣ�
ʵ�鲽�裺��1��ȡδ֪Ũ�ȵ�����20g�����ձ��У������еμ�2����ɫ��̪��Һ����ʱ��Һ��______ɫ��
��2����ȡ��������1%������������Һ50mL���ܶȽ���Ϊ1.0g/mL�����ý�ͷ�ι���εμӸ�����������Һ�������У��۲쵽______��ֹͣ�μӣ�ʣ������������Һ��10mL��
���ݴ����������������Һ����������������
ʵ�鷴˼��С������ʵ���ʱʢ�Ŵ���������ձ���ʵ�鲽���мӵ���ձ�����װ������ǰ�ڱڸ��Ž϶�ˮ���ø��ձ��������ʵ��ᵼ�½��______���ƫ��ƫС������Ӱ�족����
ʵ�鲽�裨1�����������ԣ�����ʹ��ɫ��̪��ɫ���ʴ�Ϊ���ޣ�
��ɫ��̪���������ɫ���������������ƺ����ᷢ���кͷ�Ӧ����Һ����ɫ��ɺ�ɫ��˵����Һ������һ�����������ƣ�˵������ǡ����ȫ��Ӧ���ʴ�Ϊ����ɫ�պñ�ɺ�ɫ��
���ݴ��������ĵ�����������Һ���Ϊ40mL���ۺ���40g�������������Ƶ������ǣ�40��1%=0.4��
����뷴Ӧ�Ĵ������������X��
HCl+NaOH=NaCl+H2O
36.5 40
x 0.4
| 36.5 |
| x |
| 40 |
| 0.4 |
x=0.365�ˣ�
��������������
| 0.365 |
| 20 |
������������������Ϊ1.825%��
ʵ�鷴˼���кͷ�Ӧ��ʵ���������Ӻ����������ӵķ�Ӧ���ձ��е�ˮ�������ӵ�����û����Ӱ�죬���Զ�ʵ����Ҳû��Ӱ�죬�ʴ�Ϊ����Ӱ�죮

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
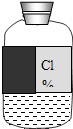
Сѧ��10����Ӧ����ϵ�д�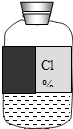 ʵ��ԱС����ѧ��ĩ����ʵ������ʱ������һƿ��ǩģ������Һ������ͼ��
ʵ��ԱС����ѧ��ĩ����ʵ������ʱ������һƿ��ǩģ������Һ������ͼ�������С��һ��̽������Һ�ijɷ֣����ⶨ������������
��һ�������벢��֤����Һ��������ʲô��
���룺���ݱ�ǩ��֪����ƿ��Һ������HCl��KCl��NaCl��
���������ӵ�����Σ�
��֤��
| ���� | ���� | ���� |
| ȡ�����μ���ɫʯ�� | ��Һ��� |
��ƿ��Һ������ |
ʵ��ԭ������1������֪Ũ�ȵ�����������Һ�����ᷴӦ����Ӧ�Ļ�ѧ����ʽΪ��
NaOH+HCl=NaCl+H20
��2�����������������ǡ����ȫ��Ӧʱ��������һ������������Һ����Һ�ͳʼ��ԣ�����ʹ��ɫ��̪��ɺ�ɫ������һ������������Һ�����ԼΪ0.05mL���Բⶨ�����Ӱ���С���ɺ��Բ��ƣ�
ʵ�鲽�裺��1��ȡδ֪Ũ�ȵ�����20g�����ձ��У������еμ�2����ɫ��̪��Һ����ʱ��Һ��
��2����ȡ��������1%������������Һ50mL���ܶȽ���Ϊ1.0g/mL�����ý�ͷ�ι���εμӸ�����������Һ�������У��۲쵽
���ݴ����������������Һ����������������
ʵ�鷴˼��С������ʵ���ʱʢ�Ŵ���������ձ���ʵ�鲽���мӵ���ձ�����װ������ǰ�ڱڸ��Ž϶�ˮ���ø��ձ��������ʵ��ᵼ�½��
 ʵ��ԱС����ѧ��ĩ����ʵ������ʱ������һƿ��ǩģ������Һ����ͼ��
ʵ��ԱС����ѧ��ĩ����ʵ������ʱ������һƿ��ǩģ������Һ����ͼ��
�����С��һ��̽������Һ�ijɷ֣����ⶨ������������
��һ�������벢��֤����Һ��������ʲô��
���룺���ݱ�ǩ��֪����ƿ��Һ������HCl��KCl��NaCl��
���������ӵ�����Σ�
��֤��
| ���� | ���� | ���� |
| ȡ�����μ���ɫʯ�� | ��Һ���______ɫ | ��ƿ��Һ������ |
�ڶ������ⶨ����Һ��������������
ʵ��ԭ������1������֪Ũ�ȵ�����������Һ�����ᷴӦ����Ӧ�Ļ�ѧ����ʽΪ��
NaOH+HCl=NaCl+H20
��2�����������������ǡ����ȫ��Ӧʱ��������һ������������Һ����Һ�ͳʼ��ԣ�����ʹ��ɫ��̪��ɺ�ɫ������һ������������Һ�����ԼΪ0.05mL���Բⶨ�����Ӱ���С���ɺ��Բ��ƣ�
ʵ�鲽�裺��1��ȡδ֪Ũ�ȵ�����20g�����ձ��У������еμ�2����ɫ��̪��Һ����ʱ��Һ��______ɫ��
��2����ȡ��������1%������������Һ50mL���ܶȽ���Ϊ1.0g/mL�����ý�ͷ�ι���εμӸ�����������Һ�������У��۲쵽______��ֹͣ�μӣ�ʣ������������Һ��10mL��
���ݴ����������������Һ����������������
ʵ�鷴˼��С������ʵ���ʱʢ�Ŵ���������ձ���ʵ�鲽���мӵ���ձ�����װ������ǰ�ڱڸ��Ž϶�ˮ���ø��ձ��������ʵ��ᵼ�½��______���ƫ��ƫС������Ӱ�족����
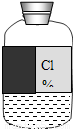 ʵ��ԱС����ѧ��ĩ����ʵ������ʱ������һƿ��ǩģ������Һ������ͼ��
ʵ��ԱС����ѧ��ĩ����ʵ������ʱ������һƿ��ǩģ������Һ������ͼ�������С��һ��̽������Һ�ijɷ֣����ⶨ������������
��һ�������벢��֤����Һ��������ʲô��
���룺���ݱ�ǩ��֪����ƿ��Һ������HCl��KCl��NaCl��
���������ӵ�����Σ�
��֤��
| ���� | ���� | ���� |
| ȡ�����μ���ɫʯ�� | ��Һ���______ɫ | ��ƿ��Һ������ |
ʵ��ԭ������1������֪Ũ�ȵ�����������Һ�����ᷴӦ����Ӧ�Ļ�ѧ����ʽΪ��
NaOH+HCl=NaCl+H2
��2�����������������ǡ����ȫ��Ӧʱ��������һ������������Һ����Һ�ͳʼ��ԣ�����ʹ��ɫ��̪��ɺ�ɫ������һ������������Һ�����ԼΪ0.05mL���Բⶨ�����Ӱ���С���ɺ��Բ��ƣ�
ʵ�鲽�裺��1��ȡδ֪Ũ�ȵ�����20g�����ձ��У������еμ�2����ɫ��̪��Һ����ʱ��Һ��______ɫ��
��2����ȡ��������1%������������Һ50mL���ܶȽ���Ϊ1.0g/mL�����ý�ͷ�ι���εμӸ�����������Һ�������У��۲쵽______��ֹͣ�μӣ�ʣ������������Һ��10mL��
���ݴ����������������Һ����������������
ʵ�鷴˼��С������ʵ���ʱʢ�Ŵ���������ձ���ʵ�鲽���мӵ���ձ�����װ������ǰ�ڱڸ��Ž϶�ˮ���ø��ձ��������ʵ��ᵼ�½��______���ƫ��ƫС������Ӱ�족����