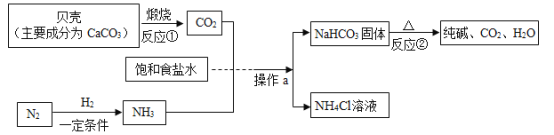
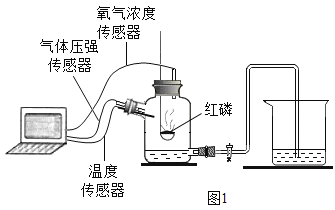
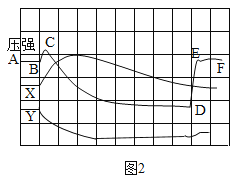
��Ŀ����
����Ŀ��ij��ѧ��ȤС���һ�����ʵ� NaOH �����˺��棬Ϊȷ����ɷ�չ������̽����������ʵ�NaOH �ɷ���ʲô��
[����] ��������Na2CO3�� ��������NaOH �� Na2CO3��
�ش��������⣺
��1������ Na2CO3����������������______�����ţ���
A�׳ƻ��
B������ˮ
C����ϡ���ᷢ����Ӧ
D�㷺���ڲ�������ֽ����֯��ϴ�Ӽ�������
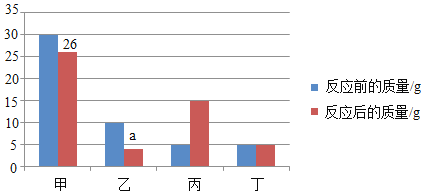
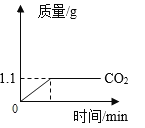
��2����ͬѧ�������������ȷ�ģ�Ϊ����֤������ȷ֪����������ʳ̶ȣ�������ͬѧȡ 5.00g����Ʒ����ϡ�����������ٸı䣬������� CO2 ��������ʱ��仯��ͼ��ʾ��
��NaOH��Һ�� pH___________7��������������=��������������
�������ֱ��ʵ� NaOH ��Һ��ϡ���ᷢ���кͷ�Ӧ�Ļ�ѧ����ʽΪ__________��
�ۼ������Ʒ�� NaOH ����������__________�����ݻ�ѧ����ʽд�������ļ��㲽�裩��
���𰸡�A �� ![]() 47%
47%
��������
��1��A��Na2CO3�׳ƴ���մʴ���
B��Na2CO3������ˮ������ȷ��
C��Na2CO3��ϡ���ᷢ����Ӧ������̼���Ȼ��ƺ�ˮ������ȷ��
D��Na2CO3�㷺���ڲ�������ֽ����֯��ϴ�Ӽ�������������ȷ��
��2����NaOH��Һ�Լ��ԣ����� pH��7��
�� NaOH ��Һ��ϡ���ᷴӦ�����Ȼ��ƺ�ˮ����Ӧ�Ļ�ѧ����ʽΪ![]() ��
��
����μӷ�ӦNa2CO3������Ϊx��
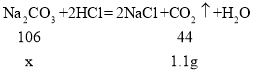
![]()
x=2.65g��
��Ʒ�� NaOH ����������![]() ��
��

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�