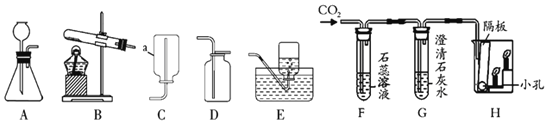
��Ŀ����
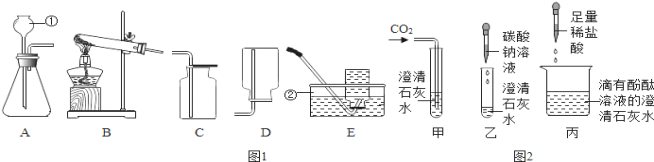
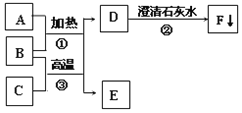
����Ŀ����һ����ɫҺ�� A װ���Թܣ��ô����ǵ�ľ�����飬�������������м���������ɫ��ĩ B ��Ѹ�ٲ����� �ݣ���������ʹ�����ǵ�ľ����ȼ������ C�������� E ��������ʢ������ C��ƿ����Һ�� D �ļ���ƿ�У����� ȼ�գ��������䣬���ɺ�ɫ���� F��
��1��д�����ǵ����ƣ�B_____��C_____�� F_____��
��2����ɫ��ĩ B �� A �ķֽⷴӦ����_____���á�
��3��д����� D �ı���ʽ_____���û�ѧʽ��ʾ��
���𰸡��������̣� ������ ������������ �����ã� 2H2O![]() 2H2��+O2����
2H2��+O2����
��������
��1��C����ʹ�����ǵ�ľ����ȼ�����壬����C��������Һ��A�ں�ɫ��ĩB���������ֽܷ���������������A�ǹ���������Һ��B�Ƕ������̣����������ڶ������̵Ĵ�����������ˮ��������������E��������ʢ������C��ƿ����Һ��D�ļ���ƿ�У����� ȼ�գ��������䣬���ɺ�ɫ����F������������ȼ�ջ������䣬���ɺ�ɫ��������������������D��ˮ��E������F��������������������֤���Ƶ���ȷ������B�Ƕ������̣�C��������F��������������
��2����ɫ��ĩB��A�ķֽⷴӦ��������ã�
��3�����D�ķ�Ӧ��ˮ��ͨ���������������������������ѧ����ʽΪ��2H2O![]() 2H2��+O2����
2H2��+O2����

 ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�����Ŀ��ʯ��ׯ28�л�ѧ��ȤС���ͬѧ��ѧϰ���꼶����ѧ���²��е����Ͽ�Ƭ��ʯ�������ʯ���γ���ʱ������������ˮ��̼��Ƶ��������ж�����̼��ˮʱ���ᷴӦ�����ܽ��Խϴ��̼����ƣ�CaCO3+CO2+H2O��Ca��HCO3��2�����뵽ʵ�����г���ʯ��ˮ�������̼��Ӧ������̼��ƣ�Ca��OH��2+CO2��CaCO3��+H2O���Գ�ʱ�������Һ��ͨ��CO2��Ӧ����Һ�е�������ɲ�����Ũ�����Ȥ��
��������⣩һ����CO2��NaOH��Һ��Ӧ������������ʲô��
���������ϣ���1��ͨ������CO2��Ӧ�Ļ�ѧ����ʽΪ��___��
��2��ͨ�����CO2����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+CO2+H2O��2NaHCO3��
��3��̼�����ζ��ǿ�����ˮ�ģ�BaCO3������ˮ��
��4��̼��������Һ�ʼ��ԡ�
��������룩��1������ΪNaOH��Na2CO3�� ��2������ΪNa2CO3����3������Ϊ___���ѧʽ������4������ΪNaHCO3��
�����ʵ�飩
ʵ�鲽�� | ʵ������ | ʵ����� |
��1���ò�����պȡ��Ӧ����Һ������pH��ֽ�� | pH��9 | ����Һ�Լ��� |
��2��ȡ��Ӧ����Һ�������Թ��У������еμӹ� ����___��Һ | ��___���� | ���루4�������� |
��3��ȡ���裨2���е��ϲ���Һ������ϡ���� | ������ð�� | ���루1���ͣ�2�� ������ |
���ó����ۣ����루3��������
�����۽�������1����ͬѧ�����ʵ�鲽�裨1���Ƕ���ģ�����Ϊ��ʵ������Ƿ���Ҫ��___��������Ҫ����������Ҫ������
��2��ͬѧ����һ�ΰ�Ŀ��Ͷ���˽̲ģ��������Ȼ�ѹǿ��Сʱ��Ca��HCO3��2��CaCO3��+CO2��+H2O����������ɷ����������NaHCO3���ķ�Ӧԭ����֮���ƣ���д��NaHCO3���ȷֽ�Ļ�ѧ����ʽ��__��
����˼Ӧ�ã�ͬѧ�ǻ�����ʵ���Ҽ��������̼���龰�����룺��������ʯ��ˮ�в���ͨ�������̼���ῴ�������������أ���������һ�£�___��