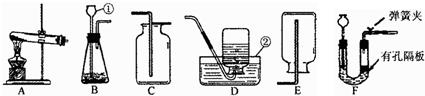
��Ŀ����
���������װ�ã��ش����⣺
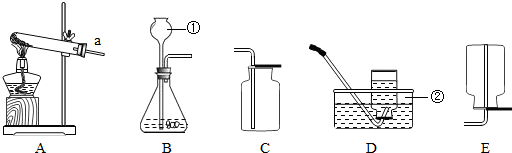
��1��д����Ţ٢ڵ��������ƣ���
��2��ʵ������ȡH2���ù�̬��п����Һ̬��ϡ�����ڳ����·�Ӧ���еģ���ѡ�õ����巢��װ����
��3���ٵ��¶˹ܿ����û���쵽Һ��������ɵĺ����
��4���ø��������ȡO2��װ��A������һ��Ķ���
��5��Ϊ�˴��Բⶨ���ȸ���������ռ���������������ɽ�Aװ������ͼF��G����װ�����ӣ���װ�ýӿڵ���ȷ����˳��Ϊ������ĸ����a��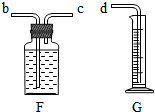

��1��д����Ţ٢ڵ��������ƣ���
����©��
����©��
����ˮ��
ˮ��
����2��ʵ������ȡH2���ù�̬��п����Һ̬��ϡ�����ڳ����·�Ӧ���еģ���ѡ�õ����巢��װ����
B
B
����дװ����ţ���������D��Eװ���ռ�H2��˵��H2������������ˮ
��������ˮ
���ܶȱȿ���С
�ܶȱȿ���С
�����ʣ���3���ٵ��¶˹ܿ����û���쵽Һ��������ɵĺ����
����ӳ���©�����ݳ�
����ӳ���©�����ݳ�
����4���ø��������ȡO2��װ��A������һ��Ķ���
���Թܿڼ�һ����
���Թܿڼ�һ����
����5��Ϊ�˴��Բⶨ���ȸ���������ռ���������������ɽ�Aװ������ͼF��G����װ�����ӣ���װ�ýӿڵ���ȷ����˳��Ϊ������ĸ����a��
c
c
��b
b
��d��
��������1���������ճ�����ѧ���������Ƽ�����;��
��2�����ݷ�Ӧ���״̬�ͷ�Ӧ������ѡ����װ�ã�����������ܶȺ��ܽ���ѡ����Ӧ���ռ�������
��3������ʵ�������ע������������ǣ�
��4�������ø��������ȡO2ʵ�������ע������������ǣ�
��5�����ݲⶨ���������ԭ��������
��2�����ݷ�Ӧ���״̬�ͷ�Ӧ������ѡ����װ�ã�����������ܶȺ��ܽ���ѡ����Ӧ���ռ�������
��3������ʵ�������ע������������ǣ�
��4�������ø��������ȡO2ʵ�������ע������������ǣ�
��5�����ݲⶨ���������ԭ��������
����⣺��1��Ҫ��dz������������ƣ��ٳ���©�� ��ˮ��
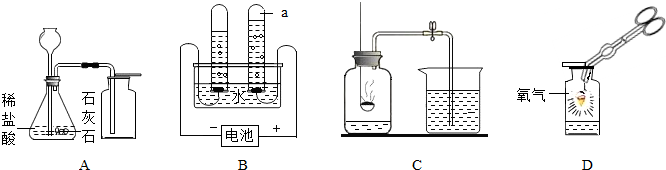
��2����ȡH2���ù�̬��п����Һ̬��ϡ�����ڳ����²���Ҫ���ȣ��ʷ���װ�õ��ص����ڷ�Ӧ���״̬�ǹ����Һ�壬����Ҫ���ȵ�B��
��D-��ˮ����E-�����ſ�����װ���ռ�H2��˵��H2���в�������ˮ���ܶȱȿ���С�����ʣ�
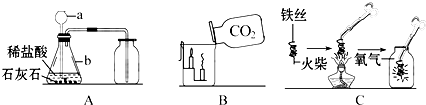
��3���¶˹ܿ����û���쵽Һ�����£���ȡ�������ӳ���©����ɢ�������У����ռ��������壻
��4���ø��������ȡ����ʱ���ŵ������Ƿ�ֹ������ؿ��������������뵼�ܣ����ø��������ȡO2��װ��A������һ��Ķ������Թܿڼ�һ������
��5����װ����������ˮ���ռ���������ͨ����Ͳ�ⶨˮ������Ӷ�֪�������������ʲ���������ͨ���̹ܽ��룬��ˮ������Ͳ��
�ʴ�Ϊ����1������©����ˮ�ۣ�
��2��B����������ˮ���ܶȱȿ���С��
��3������ӳ���©�����ݳ���
��4�����Թܿڼ�һ������
��5��c��b��
��2����ȡH2���ù�̬��п����Һ̬��ϡ�����ڳ����²���Ҫ���ȣ��ʷ���װ�õ��ص����ڷ�Ӧ���״̬�ǹ����Һ�壬����Ҫ���ȵ�B��
��D-��ˮ����E-�����ſ�����װ���ռ�H2��˵��H2���в�������ˮ���ܶȱȿ���С�����ʣ�
��3���¶˹ܿ����û���쵽Һ�����£���ȡ�������ӳ���©����ɢ�������У����ռ��������壻
��4���ø��������ȡ����ʱ���ŵ������Ƿ�ֹ������ؿ��������������뵼�ܣ����ø��������ȡO2��װ��A������һ��Ķ������Թܿڼ�һ������
��5����װ����������ˮ���ռ���������ͨ����Ͳ�ⶨˮ������Ӷ�֪�������������ʲ���������ͨ���̹ܽ��룬��ˮ������Ͳ��
�ʴ�Ϊ����1������©����ˮ�ۣ�
��2��B����������ˮ���ܶȱȿ���С��
��3������ӳ���©�����ݳ���
��4�����Թܿڼ�һ������
��5��c��b��
���������⿼���˳����������ȡ���ռ��Լ�ʵ���ע������ȣ��ѶȲ����ؼ�����ȷʵ���ԭ����

��ϰ��ϵ�д�
 ���ſ����ϵ�д�
���ſ����ϵ�д� ���Ŀ����ϵ�д�
���Ŀ����ϵ�д� ������ӱ������ͯ������ϵ�д�
������ӱ������ͯ������ϵ�д�
�����Ŀ