��Ŀ����
����Ŀ��ij��ȤС��ͬѧ��ʵ�����Ʊ�������������������̽��ʵ�顣
(1)���������������������Һ�ֽ������Ƿ��й��أ���������¶Ա�ʵ�飺
��3.0g 10%H2O2��Һ��1.0g MnO2���Ȼ����
��x g 10%H2O2��Һ��1.0g CuO���Ȼ����
����ͬ�¶��£��Ƚ�����ʵ�����O2�Ŀ�����
���з�Ӧ�Ļ�ѧ����ʽ��_________________________��
����x��ֵӦΪ_____________g��
����������������ʱȢ�죬�ɴ˵ó���ʵ�������____________________��
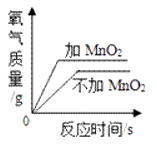
(2)��̽����Ӱ�����������Һ�ֽ��ٶȵ�ij��������ʵ�����ݼ�¼���£�
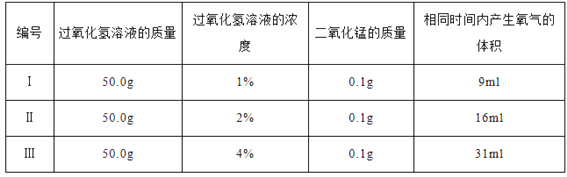
��ʵ���У�����O2�����װ����____________(����)��
ʵ����ۣ�����ͬ�����£�___________________������������Һ�ֽ��Խ�졣
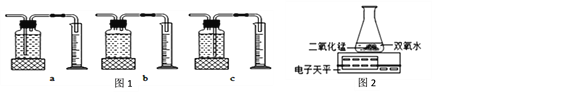
(3)������ͼ2װ�ý���ʵ�飬ͨ���Ƚ���ͬʱ����_________Ҳ�ܴﵽʵ��Ŀ�ġ�
���𰸡� 2H2O2![]() 2H2O+O2�� 3.0 ���������������������Һ�ֽ������й� C ����������ҺŨ��Խ�� ��Ӧǰ����ƽ�Ķ���֮��
2H2O+O2�� 3.0 ���������������������Һ�ֽ������й� C ����������ҺŨ��Խ�� ��Ӧǰ����ƽ�Ķ���֮��
��������(1). H2O2��Һ�� MnO2�������·ֽ�����������ˮ����Ӧ����ʽΪ��2H2O2![]() 2H2O+O2�� �� (2). �ݿ��Ʊ���ԭ������յ�����ʵ��֮�����������Һ��������ͬ������x=3.0g��(3). ��������������ʱȢ�죬�ɵó���ʵ������Ǵ��������������������Һ�ֽ������йأ� (4). ����̹ܽ������ܳ������ų��Լ�ƿ�е�ˮ�����Ա�ʵ���У�����O2�����װ����C �� (5). ����ͬ�����£�����������ҺŨ��Խ�ߣ�����������Һ�ֽ��Խ�� ��(6). �������غ㶨�ɿ�֪��Ӧǰ�����ʵ����������䣬����ʵ���У������ڼ��ٵ��������Ƿ�Ӧ�����������������ʸ��ݱȽ���ͬʱ����ƽ������ֵ��С������ӳ���ʾ����С���ٶȣ�Ҳ�ܴﵽʵ��Ŀ�ġ�
2H2O+O2�� �� (2). �ݿ��Ʊ���ԭ������յ�����ʵ��֮�����������Һ��������ͬ������x=3.0g��(3). ��������������ʱȢ�죬�ɵó���ʵ������Ǵ��������������������Һ�ֽ������йأ� (4). ����̹ܽ������ܳ������ų��Լ�ƿ�е�ˮ�����Ա�ʵ���У�����O2�����װ����C �� (5). ����ͬ�����£�����������ҺŨ��Խ�ߣ�����������Һ�ֽ��Խ�� ��(6). �������غ㶨�ɿ�֪��Ӧǰ�����ʵ����������䣬����ʵ���У������ڼ��ٵ��������Ƿ�Ӧ�����������������ʸ��ݱȽ���ͬʱ����ƽ������ֵ��С������ӳ���ʾ����С���ٶȣ�Ҳ�ܴﵽʵ��Ŀ�ġ�

����Ŀ������ʵ�鷽�����ܴﵽĿ����
ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
A | ����ˮ��˫��ˮ | ���������Ķ������� |
B | ��ȥCO2�л��е�CO | ���������ͨ���������ȵ�����ͭ |
C | ���ˮʱΪ�ӿ췴Ӧ���� | ��ˮ�м������Ƭ |
D | �����ͭ����ͭ | ��̻� |
A.AB.BC.CD.D
����Ŀ���±���һЩʳ���pH�����������θ��������ʳ�õ���
���� | ������ | ţ�� | ������ | ���� |
pH | 7.0��8.0 | 6.3��6.6 | 7.6��8.0 | 4.0~4.4 |
A.������B.����
C.������D.ţ��