��Ŀ����
����ʦ����������ͼ1��ͼ2��ʾʵ��װ��̽��ȼ�յ�������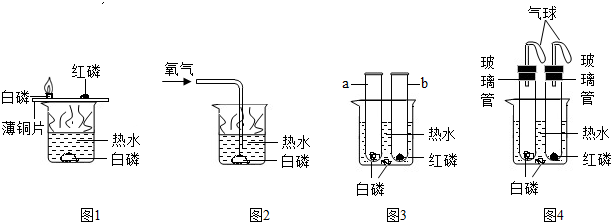
��1��ͼ1��ʾ����ʾʵ�飬��۲쵽��������
��2����ʦ��ʾͼ2��ʾʵ���Ŀ����
��3��ͨ��ͼ1��ͼ2ʵ�飬��õ��Ľ��ۣ�ȼ����������
��ͬѧ��ͨ����������֪������ȼ�ղ������������ˮ������Ӧ�������ж���ƫ���ᣨHPO3������̼��������������������ʦһ�����������ͼ3��ͼ4��ʾ��ʵ�飬��̽��ȼ�յ�������
��4������ͼ3��ʾװ�ý���ʵ�飬������д����ʵ�鱨�森
| a�Թ��а���ȼ�գ� | a�Թ��з�Ӧ����ʽ�� |
| b�Թ��к���û��ȼ�գ� | b�Թ��к���û��ȼ�յ�ԭ���ǣ� |
��ͬѧ�ǽ�ͼ3��ʾװ���е�a��b�Թܼ�����Ƥ�������̽��ȼ��������ʵ�飮ʵ�������a�Թ���ȴ���Թܿڽ���ˮ�棨���£���ȡ����Ƥ����������Һ������Թܣ�
��6�������������Ƥ��ռ�Թܵ��ݻ�����ͬѧ��Ϊ������a�Թ���Һ�������ӽ��Թ��ݻ���
| 1 |
| 5 |
| 1 |
| 5 |
��������ȼ��������̽��ʵ��ͼ1��ͼ2�Աȿ�֪ȼ����Ҫ�����ʹﵽ��ȼ��ȼ�����������¶ȣ�Ȼ��Ϊ�˷�ֹ��ȼ�պ��������ɢ�����Ⱦ����ʵ��װ�ý��������θĽ����Ľ����ͼ3ʵ����Ȼ���ܱ�֤���������ײ���ɢ�������Ľ�Ϊͼ4ʵ��װ�ã����ַ������Է�ֹ��������Ⱦ������ͬʱ��������ȼ��������������ʵ���п���������������̽��ʵ�飮
����⣺��1����ΪͭƬ�ϵİ����������Ӵ����Ż��ֻ��40�棬����ͭƬ�ϵİ���ȼ�գ��������̣���ͭƬ�ϵĺ����Ż��ܸߣ����Ժ��ײ���ȼ�գ�ˮ�а����¶���ﵽ�Ż�㣬�����������Ӵ�������ˮ�а���Ҳ����ȼ�գ�
�ʴ�Ϊ��ͭƬ�ϵİ���ȼ�գ��������̣�
��2����ͼ2ˮ�°���ͨ������ˮ�µİ���Ҳ��ȼ�գ�˵������ȼ����Ҫ������
�ʴ�Ϊ��֤������ȼ����Ҫ������
��3������������ʵ��Աȿ�֪��ȼ��ȼ����Ҫ������������������ȼ��ȼ����Ҫ���������ﵽȼ�����������¶ȣ�
�ʴ�Ϊ����ȼ��ȼ����Ҫ���������ﵽȼ�����������¶ȣ�
��4������ȼ�յĻ�ѧ����ʽΪ��4P+5O2
2P2O5��b�Թ��к��ײ�ȼ�յ�ԭ�����¶�δ�ﵽ�����Ż�㣮
�ʴ�Ϊ��4P+5O2
2P2O5���¶�δ�ﵽ�����Ż�㣮
��5��ͼ3��ͼ4��ȣ�ͼ4װ���е�С�������ռ��������ֹ������Ⱦ��
�ʴ�Ϊ���ܷ�ֹȼ�ղ�����������������ɢ��Ӱ�����彡����
��6�����������ͬ�ף���Ϊ������������ȼ���������Թ��е�������������Լռ���������
�����Խ���a�Թ���Һ�������ӽ��Թ��ݻ���
��Ҳ������ͬ�ң���Ϊ�����ײ����������IJ����Թ��е���������װ��©�������ᵼ�½���a�Թ���Һ��������һ���ӽ��Թ��ݻ���
��
�ʴ�Ϊ���ף�����Լռ���������
�����ң�ʵ���а��������㣨����װ��©���ȣ���
�ʴ�Ϊ��ͭƬ�ϵİ���ȼ�գ��������̣�
��2����ͼ2ˮ�°���ͨ������ˮ�µİ���Ҳ��ȼ�գ�˵������ȼ����Ҫ������
�ʴ�Ϊ��֤������ȼ����Ҫ������
��3������������ʵ��Աȿ�֪��ȼ��ȼ����Ҫ������������������ȼ��ȼ����Ҫ���������ﵽȼ�����������¶ȣ�
�ʴ�Ϊ����ȼ��ȼ����Ҫ���������ﵽȼ�����������¶ȣ�
��4������ȼ�յĻ�ѧ����ʽΪ��4P+5O2
| ||
�ʴ�Ϊ��4P+5O2
| ||
��5��ͼ3��ͼ4��ȣ�ͼ4װ���е�С�������ռ��������ֹ������Ⱦ��
�ʴ�Ϊ���ܷ�ֹȼ�ղ�����������������ɢ��Ӱ�����彡����
��6�����������ͬ�ף���Ϊ������������ȼ���������Թ��е�������������Լռ���������
| 1 |
| 5 |
| 1 |
| 5 |
| 1 |
| 5 |
�ʴ�Ϊ���ף�����Լռ���������
| 1 |
| 5 |
���������ⲻ�������ȼ��ȼ�յ���������ͨ��ʵ��̽����ʵ��Ľ������ʵ������ķ���������������

��ϰ��ϵ�д�
�����Ŀ
����ʦ����������ͼ1��ͼ2��ʾʵ��װ��̽��ȼ�յ�������

��1��ͼ1��ʾ����ʾʵ�飬��۲쵽��������______��
��2����ʦ��ʾͼ2��ʾʵ���Ŀ����______��
��3��ͨ��ͼ1��ͼ2ʵ�飬��õ��Ľ��ۣ�ȼ����������____________��
��ͬѧ��ͨ����������֪������ȼ�ղ������������ˮ������Ӧ�������ж���ƫ���ᣨHPO3������̼��������������������ʦһ�����������ͼ3��ͼ4��ʾ��ʵ�飬��̽��ȼ�յ�������
��4������ͼ3��ʾװ�ý���ʵ�飬������д����ʵ�鱨�森
| a�Թ��а���ȼ�գ� | a�Թ��з�Ӧ����ʽ��______�� |
| b�Թ��к���û��ȼ�գ� | b�Թ��к���û��ȼ�յ�ԭ���ǣ�______�� |
��ͬѧ�ǽ�ͼ3��ʾװ���е�a��b�Թܼ�����Ƥ�������̽��ȼ��������ʵ�飮ʵ�������a�Թ���ȴ���Թܿڽ���ˮ�棨���£���ȡ����Ƥ����������Һ������Թܣ�
��6�������������Ƥ��ռ�Թܵ��ݻ�����ͬѧ��Ϊ������a�Թ���Һ�������ӽ��Թ��ݻ���
 ����ͬѧ��Ϊ������a�Թ���Һ��������һ���ӽ��Թ��ݻ���
����ͬѧ��Ϊ������a�Թ���Һ��������һ���ӽ��Թ��ݻ���  ������ͬ��Ԥ����______����ס����ҡ�����������______��
������ͬ��Ԥ����______����ס����ҡ�����������______�� | ����ʦ����������ͼ1��ͼ2��ʾʵ��װ��̽��ȼ�յ������� | ||||
 | ||||
| (1)ͼ1��ʾ����ʾʵ�飬��۲쵽��������______________________________�� (2)��ʦ��ʾͼ2��ʾʵ���Ŀ����_____________________________________�� (3)ͨ��ͼ1��ͼ2ʵ�飬��õ��Ľ��ۣ�ȼ����������_________________________________________�� ��ͬѧ��ͨ����������֪������ȼ�ղ������������ˮ������Ӧ�������ж���ƫ����(HPO3)����̼��������������������ʦһ�����������ͼ3��ͼ4��ʾ��ʵ�飬��̽��ȼ�յ������� (4)����ͼ3��ʾװ�ý���ʵ�飬������д����ʵ�鱨�档 | ||||
| ||||
| (5) ��ͼ4װ�ý���ʵ�飬Ҳ�ܴﵽͼ3ʵ��װ�õ�ʵ��Ŀ�ģ��Ƚ�ͼ3��ͼ4��ʵ��װ�ã�����Ϊͼ4ʵ��װ�����Ե��ŵ���__________________________________�� ��ͬѧ�ǽ�ͼ3��ʾװ���е�a��b�Թܼ�����Ƥ�������̽��ȼ��������ʵ�顣ʵ�������a�Թ���ȴ���Թܿڽ���ˮ��(����)��ȡ����Ƥ����������Һ������Թܡ� (6) �����������Ƥ��ռ�Թܵ��ݻ�����ͬѧ��Ϊ������a�Թ���Һ�������ӽ��Թ��ݻ���1��5����ͬѧ��Ϊ������a�Թ���Һ��������һ���ӽ��Թ��ݻ���1��5������ͬ��Ԥ����_________(��ס����ҡ�)��������_________________________________________�� |
����ʦ����������ͼ1��ͼ2��ʾʵ��װ��̽��ȼ�յ�������

��1��ͼ1��ʾ����ʾʵ�飬��۲쵽�������� ��
��2����ʦ��ʾͼ2��ʾʵ���Ŀ���� ��
��3��ͨ��ͼ1��ͼ2ʵ�飬��õ��Ľ��ۣ�ȼ���������� ��
��ͬѧ��ͨ����������֪������ȼ�ղ������������ˮ������Ӧ�������ж���ƫ���ᣨHPO3������̼��������������������ʦһ�����������ͼ3��ͼ4��ʾ��ʵ�飬��̽��ȼ�յ�������
��4������ͼ3��ʾװ�ý���ʵ�飬������д����ʵ�鱨�森
��5����ͼ4װ�ý���ʵ�飬Ҳ�ܴﵽͼ3ʵ��װ�õ�ʵ��Ŀ�ģ��Ƚ�ͼ3��ͼ4��ʵ��װ�ã�����Ϊͼ4ʵ��װ�����Ե��ŵ��� ��
��ͬѧ�ǽ�ͼ3��ʾװ���е�a��b�Թܼ�����Ƥ�������̽��ȼ��������ʵ�飮ʵ�������a�Թ���ȴ���Թܿڽ���ˮ�棨���£���ȡ����Ƥ����������Һ������Թܣ�
��6�������������Ƥ��ռ�Թܵ��ݻ�����ͬѧ��Ϊ������a�Թ���Һ�������ӽ��Թ��ݻ��� ����ͬѧ��Ϊ������a�Թ���Һ��������һ���ӽ��Թ��ݻ���
����ͬѧ��Ϊ������a�Թ���Һ��������һ���ӽ��Թ��ݻ���  ������ͬ��Ԥ���� ����ס����ҡ����������� ��
������ͬ��Ԥ���� ����ס����ҡ����������� ��

��1��ͼ1��ʾ����ʾʵ�飬��۲쵽�������� ��
��2����ʦ��ʾͼ2��ʾʵ���Ŀ���� ��
��3��ͨ��ͼ1��ͼ2ʵ�飬��õ��Ľ��ۣ�ȼ���������� ��
��ͬѧ��ͨ����������֪������ȼ�ղ������������ˮ������Ӧ�������ж���ƫ���ᣨHPO3������̼��������������������ʦһ�����������ͼ3��ͼ4��ʾ��ʵ�飬��̽��ȼ�յ�������
��4������ͼ3��ʾװ�ý���ʵ�飬������д����ʵ�鱨�森
| a�Թ��а���ȼ�գ� | a�Թ��з�Ӧ����ʽ�� �� |
| b�Թ��к���û��ȼ�գ� | b�Թ��к���û��ȼ�յ�ԭ���ǣ� �� |
��ͬѧ�ǽ�ͼ3��ʾװ���е�a��b�Թܼ�����Ƥ�������̽��ȼ��������ʵ�飮ʵ�������a�Թ���ȴ���Թܿڽ���ˮ�棨���£���ȡ����Ƥ����������Һ������Թܣ�
��6�������������Ƥ��ռ�Թܵ��ݻ�����ͬѧ��Ϊ������a�Թ���Һ�������ӽ��Թ��ݻ���
 ����ͬѧ��Ϊ������a�Թ���Һ��������һ���ӽ��Թ��ݻ���
����ͬѧ��Ϊ������a�Թ���Һ��������һ���ӽ��Թ��ݻ���  ������ͬ��Ԥ���� ����ס����ҡ����������� ��
������ͬ��Ԥ���� ����ס����ҡ����������� ��
����ʦ����������ͼ1��ͼ2��ʾʵ��װ��̽��ȼ�յ�������

��1��ͼ1��ʾ����ʾʵ�飬��۲쵽�������� ��
��2����ʦ��ʾͼ2��ʾʵ���Ŀ���� ��
��3��ͨ��ͼ1��ͼ2ʵ�飬��õ��Ľ��ۣ�ȼ���������� ��
��ͬѧ��ͨ����������֪������ȼ�ղ������������ˮ������Ӧ�������ж���ƫ���ᣨHPO3������̼��������������������ʦһ�����������ͼ3��ͼ4��ʾ��ʵ�飬��̽��ȼ�յ�������
��4������ͼ3��ʾװ�ý���ʵ�飬������д����ʵ�鱨�森
��5����ͼ4װ�ý���ʵ�飬Ҳ�ܴﵽͼ3ʵ��װ�õ�ʵ��Ŀ�ģ��Ƚ�ͼ3��ͼ4��ʵ��װ�ã�����Ϊͼ4ʵ��װ�����Ե��ŵ��� ��
��ͬѧ�ǽ�ͼ3��ʾװ���е�a��b�Թܼ�����Ƥ�������̽��ȼ��������ʵ�飮ʵ�������a�Թ���ȴ���Թܿڽ���ˮ�棨���£���ȡ����Ƥ����������Һ������Թܣ�
��6�������������Ƥ��ռ�Թܵ��ݻ�����ͬѧ��Ϊ������a�Թ���Һ�������ӽ��Թ��ݻ��� ����ͬѧ��Ϊ������a�Թ���Һ��������һ���ӽ��Թ��ݻ���
����ͬѧ��Ϊ������a�Թ���Һ��������һ���ӽ��Թ��ݻ���  ������ͬ��Ԥ���� ����ס����ҡ����������� ��
������ͬ��Ԥ���� ����ס����ҡ����������� ��

��1��ͼ1��ʾ����ʾʵ�飬��۲쵽�������� ��
��2����ʦ��ʾͼ2��ʾʵ���Ŀ���� ��
��3��ͨ��ͼ1��ͼ2ʵ�飬��õ��Ľ��ۣ�ȼ���������� ��
��ͬѧ��ͨ����������֪������ȼ�ղ������������ˮ������Ӧ�������ж���ƫ���ᣨHPO3������̼��������������������ʦһ�����������ͼ3��ͼ4��ʾ��ʵ�飬��̽��ȼ�յ�������
��4������ͼ3��ʾװ�ý���ʵ�飬������д����ʵ�鱨�森
| a�Թ��а���ȼ�գ� | a�Թ��з�Ӧ����ʽ�� �� |
| b�Թ��к���û��ȼ�գ� | b�Թ��к���û��ȼ�յ�ԭ���ǣ� �� |
��ͬѧ�ǽ�ͼ3��ʾװ���е�a��b�Թܼ�����Ƥ�������̽��ȼ��������ʵ�飮ʵ�������a�Թ���ȴ���Թܿڽ���ˮ�棨���£���ȡ����Ƥ����������Һ������Թܣ�
��6�������������Ƥ��ռ�Թܵ��ݻ�����ͬѧ��Ϊ������a�Թ���Һ�������ӽ��Թ��ݻ���
 ����ͬѧ��Ϊ������a�Թ���Һ��������һ���ӽ��Թ��ݻ���
����ͬѧ��Ϊ������a�Թ���Һ��������һ���ӽ��Թ��ݻ���  ������ͬ��Ԥ���� ����ס����ҡ����������� ��
������ͬ��Ԥ���� ����ס����ҡ����������� ��