��Ŀ����
����Ŀ��������ˮ���ܽ�������������̼��pHΪ5.6����ʯȼ��ȼ�տ��ܲ���SO2�����յ��½����������ǿ����ͼ���γ����������һ��;����
![]()
��1�����ǰ�pH_____5.6���������������������������Ľ����Ϊ���ꡣ
��2������;���ĸ������У���Ԫ�صĻ��ϼ���_____�֡�
��3��Ϊ��������Σ�������д�ʩ��������_____������ţ��ټ�������β���ŷ� �ڽ�ֹʹ�û�ʯȼ�� �۽�ȼú��¯�̴ѼӸ� �ܿ������ܡ�̫���ܵ�����Դ
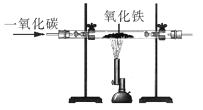
��4����ҵ�Ͽ�������������Һ���պ���������ķ�������ػ�ѧ����ʽΪ__________��
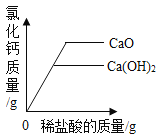
��5��ij��ȤС��ͬѧ��ʢ���Ȼ�����Һ20.8g�Ĵ��ձ��м���l0gϡ���ᣬǡ����ȫ��Ӧ�����ȥ������ʣ��Һ��������Ϊ28.47g����������Ȼ�����Һ��������������_______��
���𰸡��� 3 �٢� 2NaOH + SO2 = Na2SO3 + H2O 10%
��������
��1��pHС��5.6����ˮ�����ꡣ
��2����������Ԫ�صĻ��ϼ���0��������������Ԫ�صĻ��ϼ���+4�ۣ���������������е���Ԫ�صĻ��ϼ���+6���ʹ���3�ֻ��ϼۡ�
��3���ټ�������β���ŷſ��Լ��ٵ���������������ɣ�����ȷ��
��Ҫ����ʹ�û�ʯȼ�ϣ�����β�������ϸ�����ŷţ����ܽ�ֹʹ�û�ʯȼ�ϣ��ʴ���
�۽�ȼú��¯�̴ѼӸ߲��ܼ���������������ɣ��ʴ���
�ܿ������ܡ�̫���ܵ�����Դ���Լ���������������ɣ���ȷ����ѡ�٢ܡ�
��4�������������������Ʒ�Ӧ�����������ƺ�ˮ����Ӧ�ķ���ʽΪ��2NaOH + SO2 = Na2SO3 + H2O��
��5�������֪���������ᱵ������=20.8+10-28.47=2.33g�����Ȼ�����Һ���Ȼ���������Ϊx
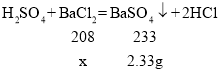
![]() ���x=2.08g
���x=2.08g
�Ȼ�����Һ��������������=![]() ��100%=10%
��100%=10%

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��С���Լ��еļ���������������Ũ�����Ȥ����ͨ���Ķ�˵�����˽�����������Ĺ���ԭ�����£����������ڲ��ķ���ɸ���������еĵ�������ȡ��Ũ��������С������̨������������ȡ���ռ���һ�����壬����ʵ���Ҷ�������о���
ʵ�����֤��������ʡ�
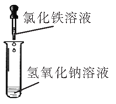
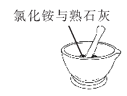
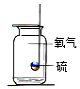
��һ��������뵽ʢ�и�����ļ���ƿ�У��۲쵽����ȼ�ո���������_____��_____�����������������ʡ���Ӧ�ı���ʽΪ_____��
ʵ��ⶨ�����������ĺ�����
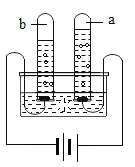
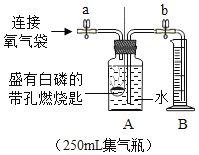
С���������ͼ��ʾװ�ã�����һ�����İ��ס���鲢ȷ��װ�õ����������ã�Ȼ�����ʵ�飬���ظ���Ρ���ʵ���������ѹǿ������������Բ��ƣ�
ʵ�鲽�裺
�ټ���ҩƷ����װ�����ӹ̶�
�ڴ�ֹˮ��a��b����A�л�������һ���������ر�ֹˮ��a��b���۲�A��ˮ���뵽B�У�B��ˮ�����Ϊ200mL��
�������۹���ȼ����
�ܴ�����Ϩ����ȴ�����£���_____����дʵ�鲽�裩���۲쵽_____������˵���ռ������岻�Ǵ�����������
�ݼ�¼B��ʣ��ˮ�������
ʵ����� | 1 | 2 | 3 | 4 | 5 |
B��ʣ��ˮ���/mL | 100 | 41 | 38 | 42 | 39 |
���ݴ�����
��1�����ϱ������У���_____�ε�����ƫ��ϴ��²����ϴ�����ԭ����ʵ��ʱ_____��
��2������4������ȡƽ��ֵ�����м��㣬�ü����������Ƶõ��������������������Ϊ_____%��
����Ŀ��������ʵ��ָ�������е�ˮ�������û������ˮ����Ҫ��������
ʵ �� װ �� |
��˿��������ȼ�� |
����������ȼ�� |
�ⶨ�������������� |
��ˮ���ռ����� |
���� | ����ƿ�е�ˮ����ȴ�����������ֹ����ƿը�� | ����ƿ�е�ˮ�����ն�������ֹ��Ⱦ���� | ��Ͳ�е�ˮ�����£����հ��� | ����ƿ�е�ˮ��ˮ�Ƚ�����ƿ�ڵĿ����ž����ڹ۲�O2��ʱ�ռ��� |
A | B | C | D |