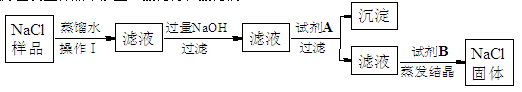
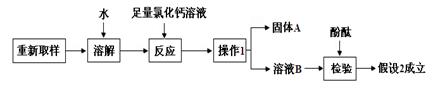
��Ŀ����
��18�֣���ҵ�ռNaOH�����нϺõ�ɱ�����������������ã�����ҵ�ռ��г���������̼���ơ�ij��ѧѧϰС��ͬѧΧ�ƹ�ҵ�ռ�չ��ϵ���о���
̽��һ����ҵ�ռ����Ƿ���̼����
���������ϡ�̼���ƺ��Ȼ����ܷ������ֽⷴӦ��
С��ָ����ѡ������ʵ��ҩƷ��̽����ϡ���ᡢ��̪��Һ��CaCl2��Һ��
��1��С��ͬѧ�������ۺ�һ����Ϊʹ�� ҩƷ���ܴﵽ̽��Ŀ�ģ������� ��
��2�������ѡ�õ�ʵ��ҩƷ�������ʵ�鱨�棺
̽�������ⶨ��ҵ�ռ���Ʒ�Ĵ���
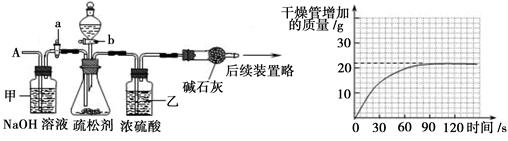
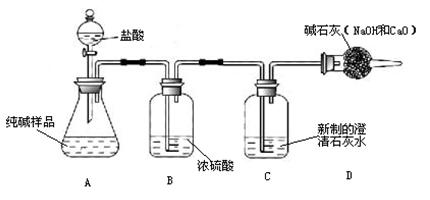
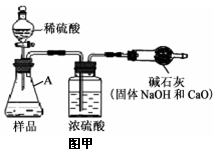
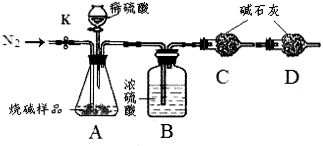
С��ͬѧ������ͼ��ʾװ�òⶨ��ҵ�ռ���Ʒ�Ĵ��ȣ��̶�װ��ʡ�ԣ�
��ʵ�鲽�衿
�ٰ�ͼ����װ�ã������װ�õ������ԣ�
�ڳ���һ�������ռ���Ʒ�����C��������
�۴��ɼ�K������N2һ��ʱ�䣻
�ܽ���װ��C��D���رյ��ɼ�K��װ��A�м���ϡ���ᣬ�����ٲ�������Ϊֹ��
���ظ�����۲������������C�����������ӡ�
�Իش�����������⣺
��1��װ��B��Ũ����������� ����֪��ʯ�ҵ���Ҫ�ɷ���CaO��NaOH����װ��D�������� ��
��2������۲�����Ŀ���� ����������û���ظ�����۵IJ�������ⶨ�ռ���Ʒ�Ĵ��Ƚ� ��ѡ�ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��3��������и����C�����������ӣ�˵�� ��д��NaOH�����ɵ����巴Ӧ�Ļ�ѧ����ʽ�� ��
��4�����в�����Ӱ�쵽����������� ������ţ���
A����ϡ�����Ϊϡ���� B��ϡ�������
C������N2ʱ��ϳ� D��ʡ��װ��D
̽��һ����ҵ�ռ����Ƿ���̼����
���������ϡ�̼���ƺ��Ȼ����ܷ������ֽⷴӦ��
С��ָ����ѡ������ʵ��ҩƷ��̽����ϡ���ᡢ��̪��Һ��CaCl2��Һ��
��1��С��ͬѧ�������ۺ�һ����Ϊʹ�� ҩƷ���ܴﵽ̽��Ŀ�ģ������� ��
��2�������ѡ�õ�ʵ��ҩƷ�������ʵ�鱨�棺
| ʵ����� | ʵ������ | ʵ����� |
| ȡ������ҵ�ռ���Ʒ�����Һ�μӹ��� | | ��ҵ�ռ��к���̼���ƣ�����ʵ������Ļ�ѧ����ʽ�� �� |
С��ͬѧ������ͼ��ʾװ�òⶨ��ҵ�ռ���Ʒ�Ĵ��ȣ��̶�װ��ʡ�ԣ�

��ʵ�鲽�衿
�ٰ�ͼ����װ�ã������װ�õ������ԣ�
�ڳ���һ�������ռ���Ʒ�����C��������
�۴��ɼ�K������N2һ��ʱ�䣻
�ܽ���װ��C��D���رյ��ɼ�K��װ��A�м���ϡ���ᣬ�����ٲ�������Ϊֹ��
���ظ�����۲������������C�����������ӡ�
�Իش�����������⣺
��1��װ��B��Ũ����������� ����֪��ʯ�ҵ���Ҫ�ɷ���CaO��NaOH����װ��D�������� ��
��2������۲�����Ŀ���� ����������û���ظ�����۵IJ�������ⶨ�ռ���Ʒ�Ĵ��Ƚ� ��ѡ�ƫ�ߡ�����ƫ�͡�����Ӱ�족����
��3��������и����C�����������ӣ�˵�� ��д��NaOH�����ɵ����巴Ӧ�Ļ�ѧ����ʽ�� ��
��4�����в�����Ӱ�쵽����������� ������ţ���
A����ϡ�����Ϊϡ���� B��ϡ�������
C������N2ʱ��ϳ� D��ʡ��װ��D
̽���һ��1����̪��Һ��1�֣� NaOH��Na2CO3��ˮ��Һ���ʼ��Զ���ʹ��ɫ��̪���ɫ(2��) ��2��ϡ���ᣨ��CaCl2��Һ�� �������ݣ�������� Na2CO3 +2HCl =" 2NaCl" + H2O +CO2������CaCl2+ Na2CO3 = CaCO3��+ 2NaCl��������ʽ3�֣�����1�֣�
̽�������1������ˮ�� ��ֹ�����е�ˮ�ֺͶ�����̼����װ��C�У�Ӱ��������
��2����װ��A��B�к��ж�����̼�Ŀ����ž�������Ӱ�������� ƫ�ߣ���1�֣�
��3�����ɵĶ�����̼����ȫ�����գ�1�֣� 2NaOH + CO2 = Na2CO3 + H2O ��3�֣�
��4��AD ��2�֣�
̽�������1������ˮ�� ��ֹ�����е�ˮ�ֺͶ�����̼����װ��C�У�Ӱ��������
��2����װ��A��B�к��ж�����̼�Ŀ����ž�������Ӱ�������� ƫ�ߣ���1�֣�
��3�����ɵĶ�����̼����ȫ�����գ�1�֣� 2NaOH + CO2 = Na2CO3 + H2O ��3�֣�
��4��AD ��2�֣�
���������̽��һ����1���������ƺ�̼���Ƶ���Һ���ʼ��ԣ�����ʹ��̪��Һ��졣���Բ����÷�̪��Һ��
��2��̼���ƿ������ᷴӦ���ɶ�����̼���壬��۲쵽���������ɡ���Ӧ�Ļ�ѧ����ʽΪ��Na2CO3 +2HCl =" 2NaCl" + H2O +CO2�����������������ᷴӦ������
�Ȼ��ƿ���̼���Ʒ�Ӧ����̼��Ƴ�������۲쵽�а�ɫ�������ɣ���Ӧ�Ļ�ѧ����ʽΪ��CaCl2+ Na2CO3 = CaCO3��+ 2NaCl���������Ʋ����Ȼ��Ʒ�Ӧ�� �ɾݴ˻ش�
̽�������ⶨ��ҵ�ռ���Ʒ�Ĵ���
��1����Ʒ�е�̼������ϡ���ᷴӦ���ɵĶ�����̼���壬Cװ���е�ҩƷ�������ɵĶ�����̼���巴Ӧ����һ������������������ö�����̼�������������Ʒ��̼���Ƶ�������A�����ɵĶ�����̼��������Һ�д���һ����ˮ�����������Cװ�û�ʹ��õĶ�����̼������������װ��B��Ũ��������������������е�ˮ��������װ��D�������Ƿ�ֹ�����еĶ�����̼�����ˮ��������װ��C�У�Ӱ����������
��2������۲�����Ŀ������ͨ��ĵ�����װ��A��B�к��ж�����̼�Ŀ����ž�������Ӱ������������������û���ظ�����۵IJ���������ܻ�ʹһ���ֶ�����̼����û�н���Dװ�ã�ʹ�������̼���Ƶ�������С���Ӷ�ʹ�ⶨ�ռ���Ʒ�Ĵ��Ƚ�ƫ�ߡ�
��3��������и����C�����������ӣ�˵�����ɵĶ�����̼����ȫ�����գ�д��NaOH�����ɵ����������̼��Ӧ������̼���ˮ����ѧ����ʽΪ��2NaOH + CO2 = Na2CO3 + H2O��
��4��A��������һ���Ļӷ��ԣ������Cװ�ûᷢ����Ӧ���Ӷ�ʹ�ⶨ��̼���������������������Ƶ�����������С��B��ϡ�������������Ʒ�е�̼��ȫ����Ӧ���Խ����Ӱ�졣C������N2ʱ��ϳ���ʹ���ɵĶ�����̼����ȫ������Cװ�ã���ʹ�����ȷ��D��ʡ��װ��D��ʹһ���ֿ�������װ��C����ʹ�ⶨ��̼���Ƶ�������������������Ƶ�����������С��

��ϰ��ϵ�д�
 ��������ϵ�д�
��������ϵ�д� ��ӡ�Ļ���ʱ����ϵ�д�
��ӡ�Ļ���ʱ����ϵ�д� ��ѧ�����ϵ�д�
��ѧ�����ϵ�д�
�����Ŀ