��Ŀ����
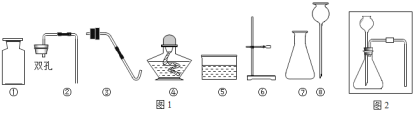
����Ŀ��ʵ��������������������ء�����ء�����ʯ��ϡ���ἰ����̨���������ʵ����Ʒ����ش���������
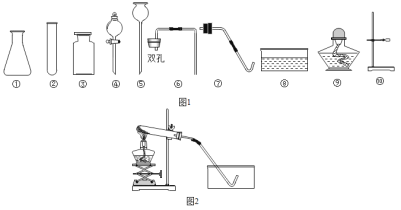
��1��ָ������������Ţ۵�����_____�����������ڻ�ѧʵ���е�һ����;��_____��
��2����������������ҩƷ����ȡij���壬�û�ѧ��Ӧ����ʽ��ʾ��ȡ������Ļ�ѧ��Ӧԭ��_____����ȡ������ѡ���������_____����������ţ���
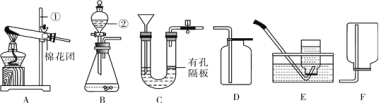
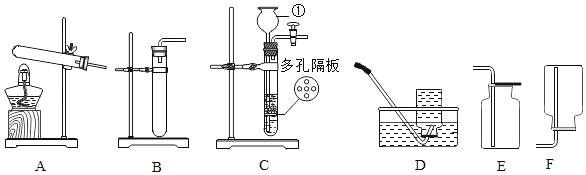
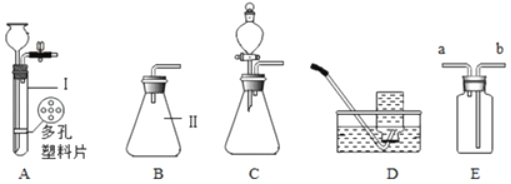
��3����ʵ��������������ҩƷ����������Ʒ��ȡ�����壬��ͼ2��������ȡ�������ʵ��װ��ͼ_____��
��4���ڸ�����ȷ��ʵ��װ�ò�©����ʵ������Ͻ�����������ɵ�ʵ�������_____�������Թܿڷ�һ������
���𰸡�����ƿ �����ռ����������� 2KMnO4![]() K2MnO4+MnO2+O2�� �ڢۢߢ���
K2MnO4+MnO2+O2�� �ڢۢߢ��� 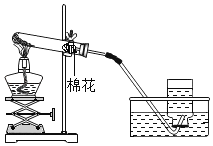 �ο����������Թ���װ�����������
�ο����������Թ���װ�����������
��������
��1������ƿ�����ռ����������壬�ʴ�Ϊ������ƿ�������ռ����������壻
��2������ø����������������Ҫ���ȣ�����������ȷֽ���������غͶ������̺�������Ҫע����ƽ����ȡ������ѡ��������ǣ��ڢۢߢ��⣻�ʴ�Ϊ��2KMnO4![]() K2MnO4+MnO2+O2�����ڢۢߢ��⣻
K2MnO4+MnO2+O2�����ڢۢߢ��⣻
��3���Թܿ�Ҫ�����ţ��ʴ�Ϊ��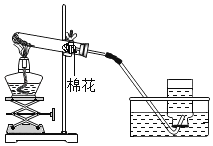
��4�����װ�õ������ԣ�������������ɵ�ʵ������ǣ��ο����������Թ���װ����������أ������Թܿڷ�һ�������ʴ�Ϊ���ο����������Թ���װ����������أ�

 ǧ�������������ĩ�����Ծ�����ϵ�д�
ǧ�������������ĩ�����Ծ�����ϵ�д�