��Ŀ����
����Ŀ����ͼ�dz��л�ѧ��������ķ���װ�ã�����Ҫ��ش��������⡣
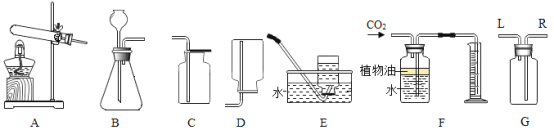
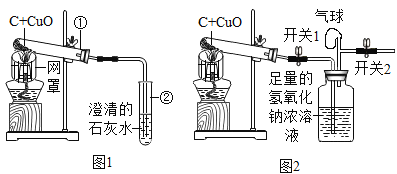
��1��ʵ������ȡ������̼��ѧ����ʽΪ__________������������Dz��Ƕ�����̼�ķ���________,��ѧ����ʽΪ____________����F���������ɶ�����̼�������������ˮ���Ϸ�һ��ֲ���͵�Ŀ����____��
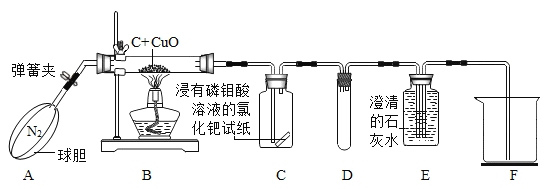
��2���ù�����������ȡ�����Ļ�ѧ����ʽ��_______����ѡ�õķ���װ����____������ţ�������Eװ���ռ�������ԭ����_______,����Gװ�����ռ������������ô�_______(�L����R��)���ܶ�ͨ�롣
��3��ʵ���ҳ���п����ϡ���ᷴӦ����ȡ������д���仯ѧ����ʽ_______��
���𰸡�CaCO3+2HCl�TCaCl2+H2O+CO2�� ������ͨ�����ʯ��ˮ�У��۲��Ƿ����� CO2+Ca��OH��2=CaCO3��+H2O ��ֹ������̼����ˮ  B ������������ˮ L Zn+H2SO4�TZnSO4+H2��
B ������������ˮ L Zn+H2SO4�TZnSO4+H2��
��������
��1��̼��ƺ����ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����ѧ����ʽΪ��CaCO3+2HCl�TCaCl2+H2O+CO2����ʵ���Ҽ��������̼���ó���ʯ��ˮ��������̼���������Ʒ�Ӧ����̼��Ƴ�����ˮ����ѧ����ʽΪ��CO2+Ca��OH��2=CaCO3��+H2O����F���������ɶ�����̼�������������ˮ���Ϸ�һ��ֲ���͵�Ŀ���ǣ���ֹ������̼����ˮ��
��2�������˫��ˮ�Ͷ��������������Ͳ���Ҫ���ȣ����÷���װ��B�����������ڶ�������������������������ˮ����������ѧ����ʽ�� ������Eװ���ռ�������ԭ���ǣ�������������ˮ������ͼG��ʾװ�����ռ������������������ܶȴ��ڿ�����Ӧ�ò����������̳����ķ�ʽ���������ſ������ռ���������Ӧ�ô�L��ͨ�룻
������Eװ���ռ�������ԭ���ǣ�������������ˮ������ͼG��ʾװ�����ռ������������������ܶȴ��ڿ�����Ӧ�ò����������̳����ķ�ʽ���������ſ������ռ���������Ӧ�ô�L��ͨ�룻
��3��ʵ��������п����ϡ�����ڳ����·�Ӧ���������仯ѧ����ʽΪ��Zn+H2SO4�TZnSO4+H2����