��Ŀ����
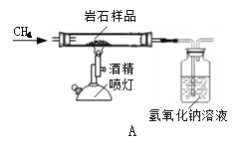
��1��ij��ѧ��ȤС�鰴����ͼ A ���вⶨ����ȼ�պ�ǰ�������仯�Ͳⶨ������ɵ�ʵ��̽�����Իش��������⣺

��ʵ����̹۲쵽�������ǣ���ƿ����___________________����;����______________________.������ȼ�����, ��ȴ���ٳ���,��ƽ��ָ�뽫��ָ��___________________ (�ƫ����ƫ�ҡ��������С�����ͬ)������ƿ�����ٳ�������ƽ��ָ�뽫��ָ��___________________��
�ڷ�Ӧ��ȫ����ȴ�����£�����ƿ������ʢ��ˮ��ˮ���У�ȡ��ƿ������� ����ƿ�е�ˮԼռ��ƿ���ݻ���___________________��
�۰���ȼ�յĻ�ѧ����ʽΪ______________________.
��2��С���������ͼ B װ����̽�������غ㶨�ɣ�ʵ�����ʱ�������е�̼���Ʒ�ĩ����ϡ�����У���ʾ Na2CO3+2HCl==2NaCl+H2O+CO2��������Ӧ��,����Ϊ��ƽ����____ (��ܡ����ܡ�)ƽ�⡣���������________________��
��3��������ʵ���֪,�ڻ�ѧ��Ӧǰ��,һ��������� ______________ (�����).
��ԭ������ ��ԭ����Ŀ�۷�������ܷ�����Ŀ��Ԫ������ �����ʵ���������
��4����˼����̽�������غ㶨��ʱ������Ӧ������μӻ����ɣ���Ӧ����_____________________��ɡ�