��Ŀ����
����Ŀ��ijѧϰС��Χ��������ʵ������ȡ�����������֡�������������������⡣
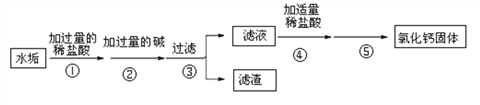
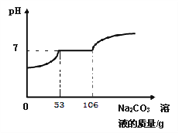
(1)ԭ��ʵ������ȡCO2�Ļ�ѧ����ʽΪ____________________������Na2CO3�����ᷴӦ��ȡCO2��ԭ����_______________________��
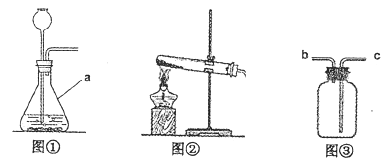
(2)ʵ���ҳ�����ˮ�����ƹ������ʯ���ڼ��ȵ��������CH4��Ӧѡͼ_________(�����)����װ�á�
(3)�ռ�װ�ã�ʵ�����ռ��ж�����SO2ʱ�������â��ռ�װ�ã�����Ӧ��_____(����ĸ)��ͨ�롣
(4)ʵ������ͼ����H2��ʹ�õ�ҩƷ��ϡ�����п����Ӧ����ʽΪ_______________��
(5)ʵ�鷴˼��ʵ������H2O2��O2�Ĺ����У����ֲ���O2�����ʺ���������鲻��װ�������Բ��ã�����Ϊ����ܵ�ԭ����____________________��
���𰸡� CaCO3+2HCl=CaCl2+H2O+CO2�� ��Ӧ���������� �� c H2SO4+Zn��ZnSO4+H2�� H2O2Ũ��ƫС��δ��MnO2(����)
��������������Ҫ�����˳�������ķ���װ�ú��ռ�װ�÷������������ȡװ�õ�ѡ���뷴Ӧ���״̬�ͷ�Ӧ�������йأ�������ռ�װ�õ�ѡ����������ܶȺ��ܽ����й���
��1������ʯ��ϡ���ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�ķ���ʽΪ��CaCO3+2HCl=CaCl2+H2O+CO2�� ��̼����Ϊ��ĩ״���ȿ�״��̼��Ʒ�Ӧ�ٶȿ�ܶ࣬�����ƣ����Բ���Na2CO3�����ᷴӦ��ȡCO2��
��2��ʵ��������ˮ�����ƹ������ʯ���ڼ��ȵ��������CH4��ѡ��ķ���װ�âڣ�
��3������������ܶȱȿ����ӳ��ܣ�c��ͨ�룬�ѿ����Ӷ̹��ų���
��4��ϡ�����п��Ӧ��������п����������Ӧ����ʽΪH2SO4+Zn��ZnSO4+H2�� ��
��5��H2O2Ũ��ƫС��δ��MnO2(����)����ʹ�����������ʺ�����ʵ������H2O2��O2�Ĺ����У����ֲ���O2�����ʺ���������鲻��װ�������Բ��ã�����ܵ�ԭ����H2O2Ũ��ƫС��δ��MnO2(����)��

 ��������������������ϵ�д�
��������������������ϵ�д�����Ŀ���ҹ��Ƽ����³ɹ��Ȼ�����гɹ������Ʊ��ǽ�������Ϊ��ҪĿ�ĵ���
A | B | C | D |
|
|
|
|
�����Ʊ�H2 | �ɹ����ɿ�ȼ�� (CH4��nH2O) | ���Ƴ�����ǿ̼�� | �ϳ�ȫ���������� (N5)6(H3O)3(NH4)4Cl |
A. A B. B C. C D. D