��Ŀ����
�±�������Ӫ���ɷ�ͳ�Ʊ���
��1����Ϊ������ֳ��һ�����ϣ��������ֻ��ţι���ף���ôţ��������ȱ����Ӫ��������dz� ֮���ά���أ�
��2������Ӫ���ɷ��еĵ�����Ӫ���������� ����ͨ�����Ϳ������Ҵ���������Ŀ�������Դ���Ҵ���������ȫȼ�շ�Ӧ�Ļ�ѧ����ʽ�ǣ� ��
��3������оҲ�д���;������ϡ�����ڼ��ȡ���ѹ�������·�Ӧ���ɵõ���Ҫ�Ļ���ԭ�Ͽ�ȩ����ȩ��C5H4O2������Է�������Ϊ ��
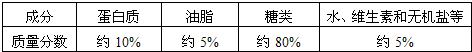
| �ɷ� | ������ | ��֬ | ���� | ά����B�� | ���� |
| �������� | Լ10% | Լ5% | Լ80% | Լ4.8% | Լ0.2% |
��2������Ӫ���ɷ��еĵ�����Ӫ����������
��3������оҲ�д���;������ϡ�����ڼ��ȡ���ѹ�������·�Ӧ���ɵõ���Ҫ�Ļ���ԭ�Ͽ�ȩ����ȩ��C5H4O2������Է�������Ϊ
��������1����������Ӫ���ɷ�ͳ�Ʊ���֪ţ��������ȱ����Ӫ��������dz�����֮���ά���ؽ��н��
��2�����ݵ�����Ӫ���������������Լ��Ҵ���������ȫȼ������ˮ�Ͷ�����̼���н��
��3�����ݿ�ȩ�Ļ�ѧʽC5H4O2����Է��������Ķ�����㣮
��2�����ݵ�����Ӫ���������������Լ��Ҵ���������ȫȼ������ˮ�Ͷ�����̼���н��
��3�����ݿ�ȩ�Ļ�ѧʽC5H4O2����Է��������Ķ�����㣮
����⣺��1������Ӫ���ɷ�ͳ�Ʊ���֪ţ��������ȱ����Ӫ��������dz�����֮���ά���أ�
��2��������Ӫ�������������࣬�Ҵ���������ȫȼ������ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽ��C2H5OH+3O2
2CO2+3H2O��
��3����ȩ�Ļ�ѧʽΪC5H4O2����Է�������=��12��5��+��1��4��+��16��2��=96��
�ʴ�Ϊ����1�����Σ���2�����ࣻC2H5OH+3O2
2CO2+3H2O����3��96��
��2��������Ӫ�������������࣬�Ҵ���������ȫȼ������ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽ��C2H5OH+3O2
| ||
��3����ȩ�Ļ�ѧʽΪC5H4O2����Է�������=��12��5��+��1��4��+��16��2��=96��
�ʴ�Ϊ����1�����Σ���2�����ࣻC2H5OH+3O2
| ||
��������ѧ��Դ�����������Ҳ�����������������������������صĻ�ѧ֪ʶ���غ����ǵ����桢���������ķ�չ�����п��ȵ�֮һ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
�±�������Ӫ���ɷ�ͳ�Ʊ���
| �ɷ� | ������ | ��֬ | ���� | ά����B�� | ���� |
| �������� | Լ10% | Լ5% | Լ80% | Լ4.8% | Լ0.2% |
��2������Ӫ���ɷ��еĵ�����Ӫ����������________����ͨ�����Ϳ������Ҵ���������Ŀ�������Դ���Ҵ���������ȫȼ�շ�Ӧ�Ļ�ѧ����ʽ�ǣ�________��
��3������оҲ�д���;������ϡ�����ڼ��ȡ���ѹ�������·�Ӧ���ɵõ���Ҫ�Ļ���ԭ�Ͽ�ȩ����ȩ��C5H4O2������Է�������Ϊ________��