��Ŀ����
������ˮ�����������������Ȼ��Դ��
��1�������к��е�����������ϡ������ȣ���һ��
��2����Ȼˮ�к����������ʣ��뾻����������ã�ijͬѧ�����ļ���ˮ������ͼ1��ʾ������̿��������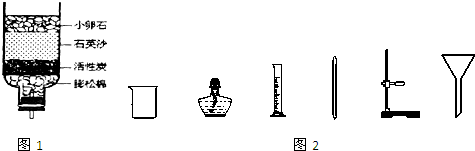
��3���������ˮ��Ӳˮ������ˮ������Ϊ����
��1�������к��е�����������ϡ������ȣ���һ��
�����
�����
����������𣩣�����ʱ�̶��벻������������Ϊ�����е���������������
��������
����2����Ȼˮ�к����������ʣ��뾻����������ã�ijͬѧ�����ļ���ˮ������ͼ1��ʾ������̿��������
����
����
��С��ʯ��ʯӢɰ������������������
����
����ʵ���ҽ��иò���������Ҫ�õ���ͼ2�����е��ƾ���
�ƾ���
����Ͳ
��Ͳ
�������ƣ���
��3���������ˮ��Ӳˮ������ˮ������Ϊ����
����ˮ
����ˮ
�����飻��������Ӳˮ���������彡����Ҫ����ˮ��Ӳ�ȣ�ͨ�����õķ��������
���
����������1�����ݿ�������ɡ����������ʷ����ش�
��2�����ݻ���̿�������ԡ����˵�ԭ�������������ش�
��3������Ӳˮ����ˮ������Ӳˮ���������������ش�
��2�����ݻ���̿�������ԡ����˵�ԭ�������������ش�
��3������Ӳˮ����ˮ������Ӳˮ���������������ش�
����⣺��1�������к��е�����������ϡ������ȣ���һ�ֻ�������ʱ�̶��벻������������Ϊ�����е������ܹ���������
��2������̿���������ԣ�����������Ϊ�������ã���ʯӢɰ�����������������Ϊ���˳������Ե����ʣ���ʵ���Ҿ�ˮ�Ĺ������ò����ƾ��ƺ���Ͳ��
��3������Ӳˮ������ˮ��������ĭ�٣���ˮ������ˮ��������ĭ�࣮���ԣ��÷���ˮ�����龻�����ˮ��Ӳˮ������ˮ��������ͨ�����ü�����еķ�����Ӳˮ������
�ʴ�Ϊ����1����������������
��2�����������ˣ��ƾ��ƣ���Ͳ��
��3������ˮ����У�
��2������̿���������ԣ�����������Ϊ�������ã���ʯӢɰ�����������������Ϊ���˳������Ե����ʣ���ʵ���Ҿ�ˮ�Ĺ������ò����ƾ��ƺ���Ͳ��
��3������Ӳˮ������ˮ��������ĭ�٣���ˮ������ˮ��������ĭ�࣮���ԣ��÷���ˮ�����龻�����ˮ��Ӳˮ������ˮ��������ͨ�����ü�����еķ�����Ӳˮ������
�ʴ�Ϊ����1����������������
��2�����������ˣ��ƾ��ƣ���Ͳ��
��3������ˮ����У�
������������Ҫ�����˿�������ɡ�ˮ�ľ��������ڻ����Ե�֪ʶ���ѶȲ���Ӧ��ǿ����֪ʶ�Ļ��ۺ�ѧϰ��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ


 ������ˮ�����������������Ȼ��Դ����������˽���٣�
������ˮ�����������������Ȼ��Դ����������˽���٣�