��Ŀ����
����Ŀ���Ͻ��������ֻ��������ϵĽ������������ǽ������ۺ϶��ɵľ��н������Ե����ʡ�һ����˵���Ͻ���۵���������κ�һ����ɽ������۵㡣�±���һЩ�����۵�����ݡ�
���� | ͭ | п | �� | Ǧ | �� | �� |
�۵�/�� | 1083 | 419.6 | 231.9 | 327.5 | 271.3 | 320.9 |
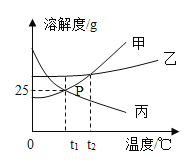
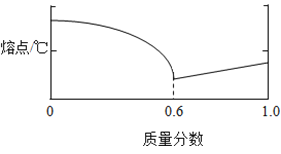
(1)Ǧ���Ͻ���ij�ֽ���������������Ͻ���۵�������ͼ��ʾ�Ĺ�ϵ�����к������ʾ����________���������������Ͻ��۵����ʱ���Ͻ���Ǧ������������Ϊ____________��
(2)����˿���顢Ǧ�������ӵȽ�����ɣ����۵�ԼΪ________��
A.20-40�� B.60-80�� C.230-250�� D.300-320��
(3)��ͭ������Ҫ�ɷ���ͭ���Ͻ�ո�µ���ͭ��������ɫ������ͭ���������ڵ��£����γ�����ɫ��ͭ�̣���Ҫ�ɷ�Cu2(OH)2CO3�������仯ѧʽ��֪��ͭ����ͭ��___________��___________��___________�������ʵĻ�ѧʽ���������õĽ����
(4)ͨ����������ĺϽ���ƷҲ�Ƚ��������⣬��д����ҵ����ϡ�����ȥ���⣨Fe2O3���Ļ�ѧ����ʽ_______________________________��
���𰸡��� 2��3 B O2 H2O CO2 Fe2O3��6HCl=2FeCl3��3H2O
��������
(1)Ǧ���Ͻ���ij�ֽ���������������Ͻ���۵�������ͼ��ʾ�Ĺ�ϵ����������������Ϊ���ʱ���۵�Ҫ����������Ϊ1��ʱ��ߣ������۵����Ǧ����������ʾ���������������������Ͻ��۵����ʱ��������������60%����Ǧ����������Ϊ40%����Ͻ���Ǧ������������Ϊ40%��60%=2��3��
(2) �Ͻ���۵��������ɳɷ��۵�Ҫ�ͣ��顢Ǧ�������������������۵���͵���231.9������Ҫѡ���۵��231.9�ͣ��Ҳ��ܺܵͣ�����Ҫ�������£�����ѡ��B��
(3) ���������غ㶨�ɿ�֪��Ӧǰ��Ԫ������䣬ͭ�̡���Ҫ�ɷ�Cu2(OH)2CO3�������仯ѧʽ��֪��ͭ���к���ͭ���⡢����̼������Ԫ�أ�ͭ����ͭ������е�������O2����ˮ��H2O���Ͷ�����̼��CO2���������õĽ����
(4)ͨ����������ĺϽ���ƷҲ�Ƚ��������⣬��ҵ����ϡ��������������Ӧ�����Ȼ�����ˮ�Ļ�ѧ����ʽ��Fe2O3��6HCl=2FeCl3��3H2O��

 A�ӽ��� ϵ�д�
A�ӽ��� ϵ�д� ȫ�Ų��Ծ�ϵ�д�
ȫ�Ų��Ծ�ϵ�д�����Ŀ����ʷ�ϵ�˿��֮·��Ҫ����Ʒ��ͨ���ޣ������һ��һ·ͬ���Ǻ����빲Ӯ��
| ��� | ���� |
�侮������ұ������������˿����������Ҷ�� | ��Ѫ��������Ƥ�������ѡ��ƹϡ����ܲ����϶����鱦���Ρ�ҩ�ġ����ϵ� | |
| ���ߡ���·���ۿڡ����������š��˵�Ȼ����豸����Դ�豸���ճ���Ʒ�� | ��Ʒ�ͺ�ʯ������Ʒ����Ȼ���� |
��1���Ŵ�ұ������ʪ����ͭ�ķ�Ӧԭ����_____���ִ���·��������Ҫ�����ĸ�������ҵ�ú�80���������ij������������������ܵõ���_____�֡�
��2����Ȼ����Ҫ�ɷ��Ǽ��飬�ڼ�����̼Ԫ������Ԫ�ص���������_____��
����Ŀ������֪ʶ���ɣ���ȫ��ȷ��һ����
A.�����仯 | B.�������� |
�ٵ��ˮ�ǽ�����ת���ɻ�ѧ�� ���ܽ�������е����ȣ��еķ��� ���ڻ�ѧ��Ӧ��ֻ��ȼ�ղ��ܷų����� | ��С�����÷Ͼɵ�ػ����� ���ظ�ʹ�����ϴ������Ϻ� �۹�ҵ�����ϡ����������ŷ� |
C.���ֺ��� | D.��Դ��Լ |
��Fe2+��һ���������Ӵ�2����λ����� ��SO3��һ��������������к���3��ԭ�� �� | �ٷϾɽ����������� ���ᳫʹ���Ҵ����� ��ʵ�����ʣҩƷ�Ż�ԭƿ |
A.AB.BC.CD.D