��Ŀ����
Ϊ�ⶨ���Ȼ��ƺ��Ȼ�����ɵĹ�����Ʒ���Ȼ��Ƶĺ�����ijͬѧ����������ʵ�飺ȡ14�˹�����Ʒ��ȫ������100��ˮ�У������õĻ����Һ�еμ�������������Ϊ10.6����̼������Һ����¼����������ͼ��ʾ�����ߣ���
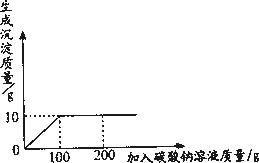
(1)���Ȼ�����̼����ǡ����ȫ��Ӧʱ�����ó���������Ϊ________�ˣ���ʱ��Ҫ�õ��������Ȼ��ƹ��壬Ӧ���еIJ�����________��
(2)��Ʒ���Ȼ��Ƶ����������Ƕ��٣�
�𰸣���1��10���Ƚ����õĻ������ˣ��ٽ��õ�����Һ���������
��2���⣺����Ʒ���Ȼ��Ƶ�����Ϊx��
CaCl2+Na2CO3 =CaCO3��+2NaCl
111 ����������100
�� x ���� 10��
111��x = 100��10�� ��֮��x =11.1��
CaCl2% = 11.1�� /14�ˡ�100%= 79.3%
��2���⣺����Ʒ���Ȼ��Ƶ�����Ϊx��
CaCl2+Na2CO3 =CaCO3��+2NaCl
111 ����������100
�� x ���� 10��
111��x = 100��10�� ��֮��x =11.1��
CaCl2% = 11.1�� /14�ˡ�100%= 79.3%

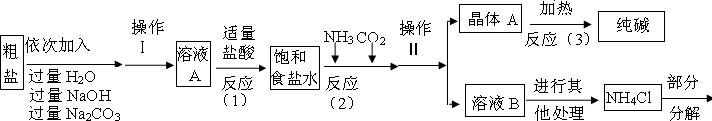
��ϰ��ϵ�д�
 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
�����Ŀ
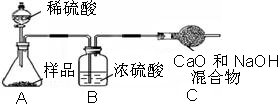 С�������������������ʸ����ȴ�����º�Ƶù�������Ϊ13.1g����Ʒ��̼���Ƶ���������Ϊ
С�������������������ʸ����ȴ�����º�Ƶù�������Ϊ13.1g����Ʒ��̼���Ƶ���������Ϊ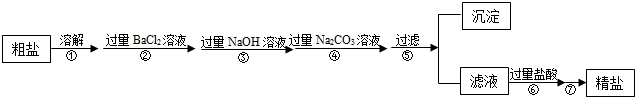
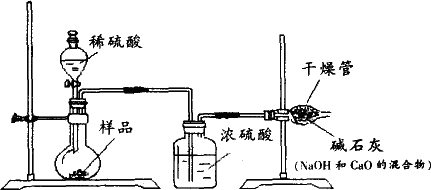

 С�������������������ʸ����ȴ�����º�Ƶù�������Ϊ13.1g����Ʒ��̼���Ƶ���������Ϊ______������Ҫ��д��������̣������·�д����
С�������������������ʸ����ȴ�����º�Ƶù�������Ϊ13.1g����Ʒ��̼���Ƶ���������Ϊ______������Ҫ��д��������̣������·�д����