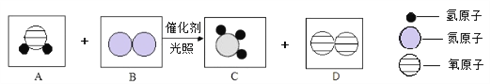
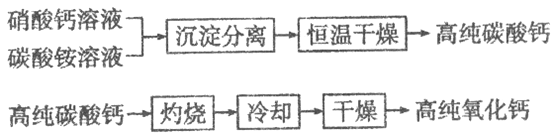
��Ŀ����
����Ŀ��ij����(Na2CO3)��Ʒ�к��������Ȼ��ơ�Ϊ�ⶨ��Ʒ��̼���Ƶ���������,�ֳ�ȡ����Ʒ11g,���뵽ʢ��50gϡ������ձ���,ǡ����ȫ��Ӧ,���Ƶ��ձ�����Һ������Ϊ56.6g��(��ܰ��ʾ��Na2CO3+2HC1�T2NaC1+H2O+CO2��)���㣺
�ٷ�Ӧ����CO2�����������___g��
�ڴ�����Ʒ��̼���Ƶ���������_____________g��
����������ձ��е���Һ���ɣ��ɵ�________g�Ȼ��ƹ���?
���𰸡� 4.4 96.4% 12.1g
���������ٸ��������غ㶨��֪����Ӧ����CO2�����������11g��50g��56.6g��4.4g��
�ڽ���贿����Ʒ��̼���Ƶ���������Ϊx�������Ȼ���Ϊy
Na2CO3+2HC1�T2NaC1+H2O+CO2��
106 `117 44
11g��x y 4.4g
![]() ��
��![]() ��x��96.4%��
��x��96.4%��
![]() ��
��![]() ��y��11.7g��
��y��11.7g��
����������ձ��е���Һ���ɣ��ɵá�11.7g��11g���v1��96.4%�w��12.1g��
�����ٷ�Ӧ����CO2�����������4.4g���ڴ�����Ʒ��̼���Ƶ�����������96.4%������������ձ��е���Һ���ɣ��ɵ�12.1g�Ȼ��ƹ��塣
�㾦�����Ӧ�������غ㶨�ɣ���ȷ��д��ѧ����ʽ��˳����ɱ������Ҫ��֤��

 ����ѵ�����⿼ϵ�д�
����ѵ�����⿼ϵ�д� �������ϵ�д�
�������ϵ�д�����Ŀ������ʵ�鷽��һ���ܴﵽʵ��Ŀ�ĵ���
ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
A | ����һƿ�����Ƿ�Ϊ | ��ȼ�ŵ�ľ������ƿ�� |
B | ���� | �ֱ��ȼ���ڻ����Ϸ���һ�����ձ� |
C | ����ʯ��ˮ�� | ����������ϡ���� |
D | �Ƚ�Zn��Cu��Ag�Ľ������ | ��Zn��Ag�ֱ���� |