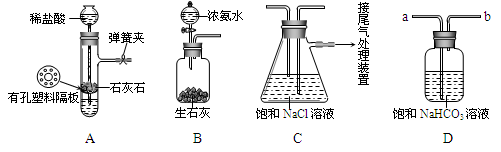
��Ŀ����
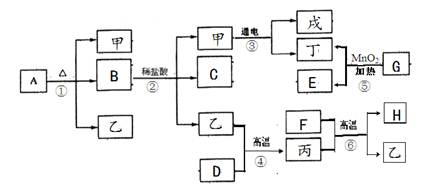
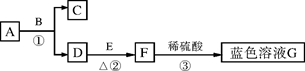
��5�֣�A��B��C��D��E��FΪ���л�ѧ�г��������ʣ�����֮��������ͼ��ʾ�ķ�Ӧ
��ϵ����������ʾ��ת������������ʾ�ܷ�����Ӧ�����ֲ�������ȥ�������к�C����
����һ����Ҫ�Ľ������ϣ�E����õ��ܼ���F������Ԫ����ɣ�����Է�������Ϊ40��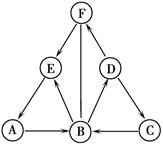
�ش��������⣺
��1��C���ʵĻ�ѧʽΪ ��
��2��D��C�Ļ�ѧ����ʽΪ ��
��3�������й�˵���У���ȷ���� ��������ţ�
��Aת��ΪB�ķ�Ӧһ���ǷֽⷴӦ
����ɫ��̪��Һ�μӵ�D����Һ��һ����ɺ�ɫ
��F��������ˮʱ����ַ�������
��C��F�ڷ����ж�������
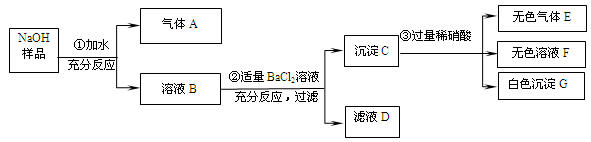
��1��CaCO3 ��2��Na2CO3+Ca(OH)2=CaCO3��+H2O (3) �ڢ�
����

��ϰ��ϵ�д�
�����Ŀ