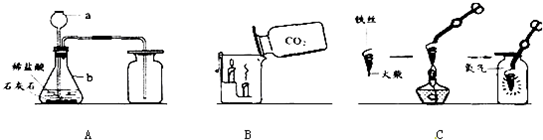
��Ŀ����
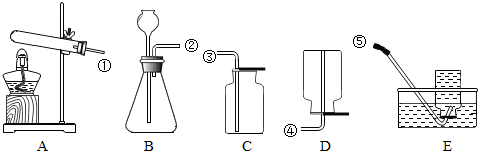
���������װ��ͼ���ش����⣺

��1�������ٵ������ǣ���
��2����B��C�����巢��װ�ã�����ȡ�������壬д����ȡ����һ������Ļ�ѧ����ʽ
��3��ʵ�����ø��������ȡO2������װ��

��1�������ٵ������ǣ���
����©��
����©��
����2����B��C�����巢��װ�ã�����ȡ�������壬д����ȡ����һ������Ļ�ѧ����ʽ
2H2O2
2H2O+O2����Zn+H2SO4 �TZnSO4+H2���� CaCO3+2HCl�TCaCl2+H2O+CO2��
| ||
2H2O2
2H2O+O2����Zn+H2SO4 �TZnSO4+H2���� CaCO3+2HCl�TCaCl2+H2O+CO2��
����B��ȣ�C���ŵ���
| ||
��������Һ��
��������Һ��
����3��ʵ�����ø��������ȡO2������װ��
A
A
��Ϊ����װ�ã������Eװ���ռ�O2���ռ����Ӧ�Ƚ������ܴ�ˮ�г���ٳ����ƾ��ƣ�ԭ������ֹˮ�ӵ��ܻ��������Թ�����
��ֹˮ�ӵ��ܻ��������Թ�����
�����������ݳ�������ķ�Ӧ��״̬������+���壩�ͷ�Ӧ��������Ҫ���ȣ���ʵ������ȡ����ѡȡ���巢��װ��A�������������ȡ�����ʵ����ϣ�Ӧ�Ƚ����ܴ�ˮ���г�������Ϩ�ƾ���
����⣺��1����������Ϊ����©��
��2��BCΪ��Һ������װ�ã�������ȡ�����������������̼����ѧ����ʽΪ��2H2O2
2H2O+O2����Zn+H2SO4 �TZnSO4+H2���� CaCO3+2HCl�TCaCl2+H2O+CO2�� Cװ�����ó���©������������Һ�壻
��3����������ǹ��壬��Ӧ�����Ǽ��ȣ����Ӧѡ��Aװ�ã�����ˮ���ռ�����ʱ������Ϩ��ƾ��ƣ����Թ���ѹǿ��С������ѹ�Ὣˮ�ص���ѹ���ȵ��Թܣ�ʹ�Թ�ը�ѣ�
�ʴ�Ϊ����1������©�� ��2��2H2O2
2H2O+O2����Zn+H2SO4 =ZnSO4+H2���� CaCO3+2HCl�TCaCl2+H2O+CO2�� ��������Һ�壨3��A ��ֹˮ�ӵ��ܻ��������Թ�����
��2��BCΪ��Һ������װ�ã�������ȡ�����������������̼����ѧ����ʽΪ��2H2O2
| ||
��3����������ǹ��壬��Ӧ�����Ǽ��ȣ����Ӧѡ��Aװ�ã�����ˮ���ռ�����ʱ������Ϩ��ƾ��ƣ����Թ���ѹǿ��С������ѹ�Ὣˮ�ص���ѹ���ȵ��Թܣ�ʹ�Թ�ը�ѣ�
�ʴ�Ϊ����1������©�� ��2��2H2O2
| ||
������������Ҫ���鳣������ķ���װ�ú��ռ�װ�õ�ѡȡ����������Ҫ��ȷ��д��ѧʽ�ͻ�ѧ����ʽ��

��ϰ��ϵ�д�
 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
�����Ŀ