��Ŀ����
 ijУ��ѧ��ȤС��ͬѧ�������غ㶨�ɽ���ʵ��̽��������ͬѧ���ڿ�����ȼ��þ����ʵ����̽��������ͬѧ��ͼ������̽�����ش��������⣺
ijУ��ѧ��ȤС��ͬѧ�������غ㶨�ɽ���ʵ��̽��������ͬѧ���ڿ�����ȼ��þ����ʵ����̽��������ͬѧ��ͼ������̽�����ش��������⣺��1��þ��ȼ�յĻ�ѧ����ʽΪ
��2������ͬѧ��þ��ȼ�պ�������ʯ�����Ϲ�������������ֱȷ�Ӧǰþ��������Ҫ�ᣮ����������п��ܵ�ԭ��
������������ͬѧ��ȼ�յ�þ���Ϸ������֣�ʹ�гֵ�þ����ȫȼ�ղ�ʹ������ȫ���ռ�������������Ƶõ�������ԭþ��������
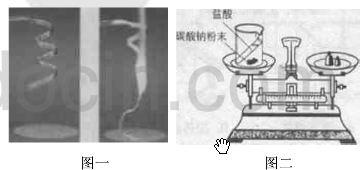
��3����֪̼������������Һ����ѧʽΪHCl����Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼��д���˱仯�Ļ�ѧ����ʽ
��4��ͨ����������ʵ�飬���õ���ʾ��
��������1������þ���ڿ�����ȼ����������þ���н��
��2�����ݸ�ʵ���ע��������н��
��3������̼������������Һ����ѧʽΪHCl����Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���н��
��4����������ʵ���֪��֤�����غ㶨��ʱ������������μӻ����������ɵķ�Ӧ������ܷ�������н��н��
��2�����ݸ�ʵ���ע��������н��
��3������̼������������Һ����ѧʽΪHCl����Ӧ�����Ȼ��ơ�ˮ�Ͷ�����̼���н��
��4����������ʵ���֪��֤�����غ㶨��ʱ������������μӻ����������ɵķ�Ӧ������ܷ�������н��н��
����⣺��1��þ���ڿ�����ȼ����������þ����Ӧ�Ļ�ѧ����ʽΪ��2Mg+O2
2MgO���ʴ�Ϊ��2Mg+O2
2MgO��
��2������ͬѧ��þ��ȼ�պ�������ʯ�����Ϲ�������������ֱȷ�Ӧǰþ��������Ҫ�ᣮ���п��ܵ�ԭ��þ����ȫȼ��ʱ���ܲ��������ݵ������У������ȷ�Ӧǰ�٣������ڼ�ȡþ����Ӧ�������ǯ��մ��������Ӧ�ʹʣ����������٣��������ȼ�յ�þ���Ϸ������֣�ʹ�гֵ�þ����ȫȼ�ղ�ʹ������ȫ���ռ�������������Ƶõ�������ԭþ�������ȴ�ԭ���Dzμӷ�Ӧ��þ�����������������������ɵ�����þ���������������ɵ�����þ����������þ���������ʴ�Ϊ��þ����ȫȼ��ʱ���ܲ��������ݵ������У������ȷ�Ӧǰ�٣������ڼ�ȡþ����Ӧ�������ǯ��մ��������Ӧ�ʹʣ����������٣�����μӷ�Ӧ��þ�����������������������ɵ�����þ���������������ɵ�����þ����������þ��������
��3��̼���������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl�T2NaCl+H2O+CO2������Ϊ���ɵĶ�����̼�����ݳ������Թ۲쵽��ƽ����ƫת���ʴ�Ϊ��Na2CO3+2HCl�T2NaCl+H2O+CO2��������ƫת��
��4��ͨ����������ʵ�飬��õ���ʾ����֤�����غ㶨��ʱ������������μӻ����������ɵķ�Ӧ������ܷ�������н��У�����п�ѧ̽��ʱ��Ҫ�������������ʣ����ʴ�Ϊ����֤�����غ㶨��ʱ������������μӻ����������ɵķ�Ӧ������ܷ�������н��У�����п�ѧ̽��ʱ��Ҫ�������������ʣ���
| ||
| ||
��2������ͬѧ��þ��ȼ�պ�������ʯ�����Ϲ�������������ֱȷ�Ӧǰþ��������Ҫ�ᣮ���п��ܵ�ԭ��þ����ȫȼ��ʱ���ܲ��������ݵ������У������ȷ�Ӧǰ�٣������ڼ�ȡþ����Ӧ�������ǯ��մ��������Ӧ�ʹʣ����������٣��������ȼ�յ�þ���Ϸ������֣�ʹ�гֵ�þ����ȫȼ�ղ�ʹ������ȫ���ռ�������������Ƶõ�������ԭþ�������ȴ�ԭ���Dzμӷ�Ӧ��þ�����������������������ɵ�����þ���������������ɵ�����þ����������þ���������ʴ�Ϊ��þ����ȫȼ��ʱ���ܲ��������ݵ������У������ȷ�Ӧǰ�٣������ڼ�ȡþ����Ӧ�������ǯ��մ��������Ӧ�ʹʣ����������٣�����μӷ�Ӧ��þ�����������������������ɵ�����þ���������������ɵ�����þ����������þ��������
��3��̼���������ᷴӦ�����Ȼ��ơ�ˮ�Ͷ�����̼����Ӧ�Ļ�ѧ����ʽΪ��Na2CO3+2HCl�T2NaCl+H2O+CO2������Ϊ���ɵĶ�����̼�����ݳ������Թ۲쵽��ƽ����ƫת���ʴ�Ϊ��Na2CO3+2HCl�T2NaCl+H2O+CO2��������ƫת��
��4��ͨ����������ʵ�飬��õ���ʾ����֤�����غ㶨��ʱ������������μӻ����������ɵķ�Ӧ������ܷ�������н��У�����п�ѧ̽��ʱ��Ҫ�������������ʣ����ʴ�Ϊ����֤�����غ㶨��ʱ������������μӻ����������ɵķ�Ӧ������ܷ�������н��У�����п�ѧ̽��ʱ��Ҫ�������������ʣ���
����������Ϣ�����л�ȡ��������˼·�ͷ���������Ҫ��������������ϵļ�ֵ��Ȼ���������������˼����

��ϰ��ϵ�д�
�����Ŀ
 ��2012?������һģ��ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽10gˮ���У�����CO2����������ͼ��ʾ��
��2012?������һģ��ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽10gˮ���У�����CO2����������ͼ��ʾ��  ijУ��ѧ��ȤС��ͬѧ��ʵ��ⶨһ�����ʯ��̼��Ƶ��������������dz�ȡ�Ĵ���ʯ����Ϊ12.5g���������������ϡ�����У���������������ͼ��ʾ������ô���ʯ��̼��Ƶ�����������������ʯ�г�̼���֮����������ʲ������ᷢ����Ӧ��
ijУ��ѧ��ȤС��ͬѧ��ʵ��ⶨһ�����ʯ��̼��Ƶ��������������dz�ȡ�Ĵ���ʯ����Ϊ12.5g���������������ϡ�����У���������������ͼ��ʾ������ô���ʯ��̼��Ƶ�����������������ʯ�г�̼���֮����������ʲ������ᷢ����Ӧ�� ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽12.5gˮ������CO2����������ͼ��ʾ��
ijУ��ѧ��ȤС��ͬѧ���֣�����ʹ�õ���ˮ���ײ���һ��ˮ����ˮ������Ҫ�ɷ���̼��ƺ�������þ������Ϊ�˲ⶨˮ����̼��Ƶĺ�������������������Ϊ10%��������뵽12.5gˮ������CO2����������ͼ��ʾ��