��Ŀ����
����Ŀ��С��ͬѧΪ̽������Ļ�ѧ���ʣ���������̽��ʵ��:
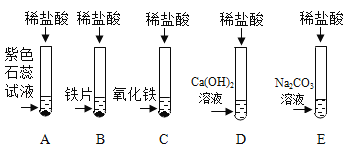
(1)�Թ�C�з�Ӧ�Ļ�ѧ����ʽΪ______________��
(2)С��ͬѧ�ѷ�Ӧ��D��E�Թ��е���Һ����һ���ྻ���ձ���,�۲쵽�������ݲ��������а�ɫ�������ɡ����ˣ��õ���ɫ��������ɫ��Һ��
������ʵ������ɵã�ԭD�Թ��з�Ӧ����Һ��������______________��
С��ͬѧ��̽����ɫ��Һ�е����ʳɷ�:
[�������]��ɫ��Һ��������ʲô?
[��������]�����: NaCl;
�����:______________��
�����:_____________��
[ʵ��̽��]
ʵ�鲽�� | ʵ������ | ʵ����� |
��ȡ������Һ���Թ���,�μ�����̼������Һ | ���������� | ����ڲ����� |
����ȡ������Һ���Թ��У��μ��������� | _____________�� | ����۳��� |
[��չ����]����η�����Ӧ��������_____________��
���𰸡�![]() HCl��CaCl2 NaCl��CaCl2 NaCl��Na2CO3 �����ݲ��� �г�������ˮ���ɻ���ϸ��ֽⷴӦ������
HCl��CaCl2 NaCl��CaCl2 NaCl��Na2CO3 �����ݲ��� �г�������ˮ���ɻ���ϸ��ֽⷴӦ������
��������
��1�������������ᷴӦ�����Ȼ�����ˮ����ѧ����ʽΪ��6HCl+Fe2O3�T2FeCl3+3H2O��
��2���ѷ�Ӧ��D��E�Թ��еķ�Һ����һ���ྻ���ձ��У��۲쵽�������ݲ��������а�ɫ�������ɣ���˵��E�Թ���̼������Һ������D�е��������������ԭD�Թ��з�Ӧ����Һ��������HCl��CaCl2��
[��������]E�Թ���̼������Һ������D�е����������D�е����ʺ���������Ȼ��ƣ�����ǡ����ȫ��Ӧ���������е�һ�����ʹ��������Բ���٣�NaCl�� �����NaCl��CaCl2�� ����ۣ�NaCl��Na2CO3��
[ʵ��̽��]̼���ƺ����ᷴӦ�����ɶ�����̼������
ʵ�鲽�� | ʵ������ | ʵ����� |
��ȡ������Һ���Թ��е� | ���������� | ����ڲ����� |
����ȡ������Һ���Թ��У� | �����ݲ��� | ����۳��� |
[��չ����]����η�����Ӧ�������ǣ��г�������ˮ���ɻ���ϸ��ֽⷴӦ��������
�ʴ�Ϊ����1��6HCl+Fe2O3�T2FeCl3+3H2O��
��2��HCl��CaCl2��
[��������]����ڣ�NaCl��CaCl2��
����ۣ�NaCl��Na2CO3��
[ʵ��̽��]�����ݲ�����
[��չ����]�г�������ˮ���ɻ���ϸ��ֽⷴӦ��������

 ȫ�ܲ����ĩС״Ԫϵ�д�
ȫ�ܲ����ĩС״Ԫϵ�д�����Ŀ���������и������ʰ�ͼ�м�ͷ����ͨ��һ������ʵ��ͼ����ʾת������
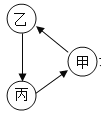
�� | �� | �� | |
A | Ca(OH)2 | CaCO3 | H2O |
B | Fe | Fe3O4 | CO2 |
C | H2O2 | H2O | O2 |
D | CO2 | CO | H2O |
A.AB.BC.CD.D
����Ŀ����ѧͨ��ʵ���о����ʵ���ɡ����ʺͱ仯���ɡ�
�� | �� | �� | �� |
|
|
|
|
̽��Ӱ�������ܽ��Ե����� | ̽��ˮ����� | ̽�� CO2 ���� | ̽��ȼ������ |
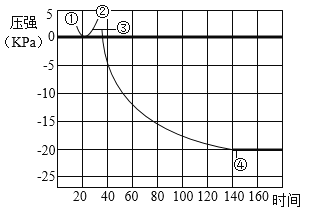
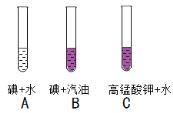
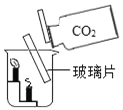
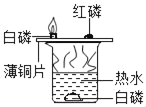
��1��ʵ����У����� B �й�����ܣ�A �м������ܣ�˵��Ӱ�������ܽ��Ե�������_____������ C �Թ�ʵ�飬��̽����Ӱ��������_____��
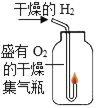
��2��ʵ�����У��۲쵽����ƿ�ڱ���_____���������Եó����ۣ�ˮ��_____����Ԫ����ɡ�
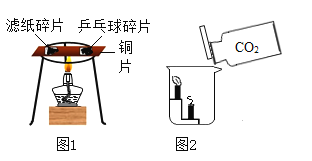
��3��ʵ����У��۲쵽��������_____�������˶�����̼_____���ʡ�
��4��ʵ�鶡�У���ȼ�յĻ�ѧ����ʽ_____��������ˮ��������_____��