��Ŀ����
ʵ����̽��ij�о���ѧϰС���Թ�������������Ϊ�о�����̽����ɷֲ����ս�����
��������֪���÷��������������ܻ�����Fe��OH��3��Fe2O3��Fe��OH��3������ˮ�����ȷ����ֽⷴӦ��2Fe��OH��3��Fe2O3+3H2O�������������ʲ�������Ӧ��
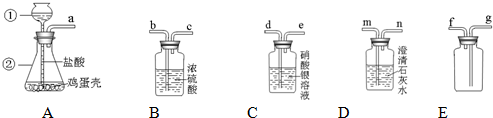
��1������ݴ�������ڷ�����ɷ֣����ʳ��⣩�ļ��裺
a��
��2��ʵ�������
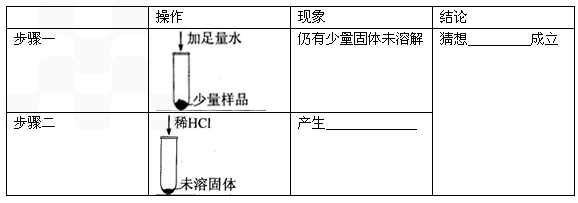
��ȡ������������Ʒɹ�ɣ���������Ϊm1�ˣ�
��������Ʒ����������ּ��Ȳ��ڸ���������ȴ�Ƶ�ʣ���������Ϊm2�ˣ���ش�
��ml=m2ʱ����Ʒ��һ����
| 80 | 107 |
��3�����ս���
��a������ȷ��������Ʒ�е�Fe2O3�辭��������Ҫ���裺Fe2O3����Ʒ�У���FeCl3��Һ��Fe��OH��3��Fe2O3����д��ǰ������Ӧ�Ļ�ѧ����ʽ��
��������������ṩ����Ϣ���÷��������������ܻ�����Fe��OH��3��Fe2O3���ɴ����������ʽ��в²⣬Fe��OH��3������ˮ�����ȷ����ֽⷴӦ�����¹����������٣����Ⱥ���������Ƿ����仯��ȷ���Ƿ������������ı�־�����������������ᷴӦ�����κ�ˮ�����������Ȼ�����������Լ��������Ʒ������ֽⷴӦ��
����⣺��1���÷��������������ܻ�����Fe��OH��3��Fe2O3�������ֻ����������������������Ҳ�п������������ʶ����У����Ա����Ϊ��Fe2O3��Fe��OH��3��Fe��OH��3��Fe2O3��
��2��Fe��OH��3���ȷ����ֽⷴӦ�����¹����������٣���������������������Ⱥ�����������䣬��������������������Ⱥ����������С�����Ա����Ϊ��Fe2O3������
��3�����������������ᷴӦ�����Ȼ�����ˮ���Ȼ��������������Ʒ�Ӧ�������������������Ȼ��ƣ����Ա����Ϊ��Fe2O3+6HCl=2FeCl3+3H2O��FeCl3+3NaOH=Fe��OH��3��+3NaCl��
��2��Fe��OH��3���ȷ����ֽⷴӦ�����¹����������٣���������������������Ⱥ�����������䣬��������������������Ⱥ����������С�����Ա����Ϊ��Fe2O3������
��3�����������������ᷴӦ�����Ȼ�����ˮ���Ȼ��������������Ʒ�Ӧ�������������������Ȼ��ƣ����Ա����Ϊ��Fe2O3+6HCl=2FeCl3+3H2O��FeCl3+3NaOH=Fe��OH��3��+3NaCl��
���������⿼���˷Ͼɽ����Ļ��գ��Լ������֮��ĸ��ֽⷴӦ����ɴ��⣬�����������е�֪ʶ���У�

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
��6�֣�ij�о���ѧϰС��������ϵ�֪��
��̿����Ҫ�ɷ���̼���ʣ����ʲ���ˮ��Ӧ����ˮ�����ڸ��������·�Ӧ���ܲ���һ���׳�Ϊˮú��������ȼ�ϣ�ˮú���в���������л����
Ϊ̽��ˮú���ijɷݣ���С�鿪չ�����»��
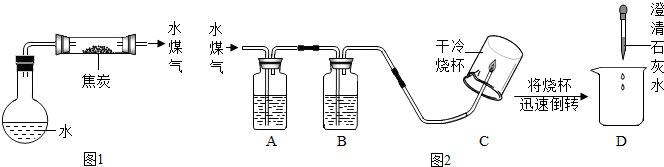
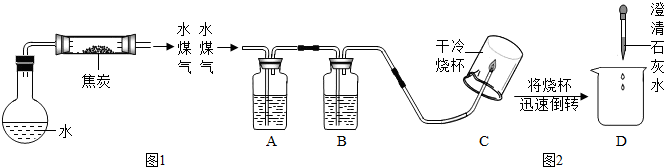
��1����ƽ�̿��ˮ������Ӧ��װ�ã���ͼ1��ͼ�мг������ͼ���������ʡ�ԣ���
��2���������裺������ѧ֪ʶ������ˮú����һ������ˮ���������ܺ���H2��CO��CO2��
��3��ʵ��̽����ͬѧ��Ϊ̽��ˮú���ijɷݣ����������ʵ��װ��(ͼ2)������ʵ�顣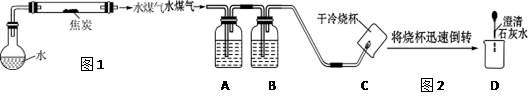
�����ͼ2��ʾװ�õ�ʵ��ԭ������д�±��еĿո�
| ������� | ��ʢ�Լ������� | �Լ���װ�õ����� |
| A | | �����Ƿ���CO2 |
| B | Ũ���� | ����ˮ�� |
| C | | |
| D | | |
����֤��CO2���ڵ������� ������֤��CO���ڵ������� ��
����֤��H2���ڵ������� ��