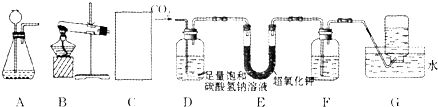��Ŀ����
ij�о���ѧϰС��������ϵ�֪��
��̿����Ҫ�ɷ���̼���ʣ����ʲ���ˮ��Ӧ����ˮ�����ڸ��������·�Ӧ���ܲ���һ���׳�Ϊˮú��������ȼ�ϣ�ˮú���в���������л����
Ϊ̽��ˮú���ijɷݣ���С�鿪չ�����»��
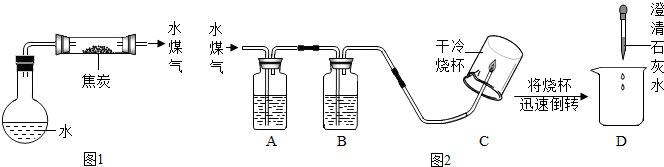
��1����ƽ�̿��ˮ������Ӧ��װ�ã���ͼ1��ͼ�мг������ͼ���������ʡ�ԣ���
��2���������裺������ѧ֪ʶ������ˮú����һ������ˮ���������ܺ���H2��CO��CO2��
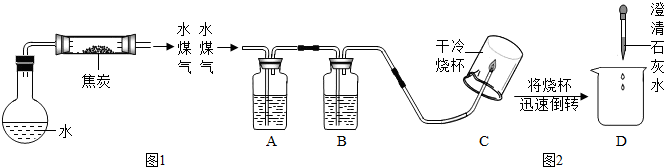
��3��ʵ��̽����ͬѧ��Ϊ̽��ˮú���ijɷݣ����������ʵ��װ�ã�ͼ2��������ʵ�飮
�����ͼ2��ʾװ�õ�ʵ��ԭ������д�±��еĿո�
��4�����������ʵ��֤��ˮú������H2��CO��CO2��
����֤��CO2���ڵ�������
����֤��CO���ڵ�������
����֤��H2���ڵ�������

��̿����Ҫ�ɷ���̼���ʣ����ʲ���ˮ��Ӧ����ˮ�����ڸ��������·�Ӧ���ܲ���һ���׳�Ϊˮú��������ȼ�ϣ�ˮú���в���������л����
Ϊ̽��ˮú���ijɷݣ���С�鿪չ�����»��
��1����ƽ�̿��ˮ������Ӧ��װ�ã���ͼ1��ͼ�мг������ͼ���������ʡ�ԣ���
��2���������裺������ѧ֪ʶ������ˮú����һ������ˮ���������ܺ���H2��CO��CO2��
��3��ʵ��̽����ͬѧ��Ϊ̽��ˮú���ijɷݣ����������ʵ��װ�ã�ͼ2��������ʵ�飮
�����ͼ2��ʾװ�õ�ʵ��ԭ������д�±��еĿո�
| ������� | ��ʢ�Լ������� | �Լ���װ�õ����� |
| A | ����ʯ��ˮ ����ʯ��ˮ |
�����Ƿ���CO2 |
| B | Ũ���� | ����ˮ�� |
| C | ����ȼ������ˮ�������ձ��ڱ�����ˮ����֣���֤��H2���� ����ȼ������ˮ�������ձ��ڱ�����ˮ����֣���֤��H2���� | |
| D | һ����̼ȼ�����ɶ�����̼��������̼��ʹ����ʯ��ˮ����ǣ���֤��CO���� һ����̼ȼ�����ɶ�����̼��������̼��ʹ����ʯ��ˮ����ǣ���֤��CO���� |
����֤��CO2���ڵ�������
A�г���ʯ��ˮ�����
A�г���ʯ��ˮ�����
������֤��CO���ڵ�������
D�г���ʯ��ˮ�����
D�г���ʯ��ˮ�����
������֤��H2���ڵ�������
C���ձ��ڱ�����ˮ�����
C���ձ��ڱ�����ˮ�����
����������3�����ݶ�����̼��ʹ����ʯ��ˮ����ǣ������ж�A��ʢ�ŵ��Լ��dz���ʯ��ˮ����������ȼ������ˮ�����Կ����ձ��ڱ�����ˮ����֣�һ����̼ȼ�����ɶ�����̼��������̼��ʹ����ʯ��ˮ����ǣ�
��4�����ݶ�����̼��ʹ����ʯ��ˮ����ǿ���֤��������̼�Ĵ��ڣ�����һ����̼ȼ�����ɶ�����̼��������̼��ʹ����ʯ��ˮ�������֤��CO���ڣ���������ȼ������ˮ�������ձ��ڱ�����ˮ�������֤��H2���ڣ�
��4�����ݶ�����̼��ʹ����ʯ��ˮ����ǿ���֤��������̼�Ĵ��ڣ�����һ����̼ȼ�����ɶ�����̼��������̼��ʹ����ʯ��ˮ�������֤��CO���ڣ���������ȼ������ˮ�������ձ��ڱ�����ˮ�������֤��H2���ڣ�
����⣺��3��������̼��ʹ����ʯ��ˮ����ǿ����ж�A��ʢ�ŵ��Լ��dz���ʯ��ˮ������ȼ������ˮ�Լ�һ����̼ȼ�����ɶ�����̼��������̼��ʹ����ʯ��ˮ����ǽ��н��
��4��������̼��ʹ����ʯ��ˮ����ǿ���֤��������̼�Ĵ��ڣ�һ����̼ȼ�����ɶ�����̼��������̼��ʹ����ʯ��ˮ�������֤��CO���ڣ���Ϊ����ȼ������ˮ������C���ձ��ڱ�����ˮ����֣���֤��H2���ڣ�
�ʴ�Ϊ����3��
��4����A�г���ʯ��ˮ����ǣ���D�г���ʯ��ˮ����ǣ���C���ձ��ڱ�����ˮ����֣�
��4��������̼��ʹ����ʯ��ˮ����ǿ���֤��������̼�Ĵ��ڣ�һ����̼ȼ�����ɶ�����̼��������̼��ʹ����ʯ��ˮ�������֤��CO���ڣ���Ϊ����ȼ������ˮ������C���ձ��ڱ�����ˮ����֣���֤��H2���ڣ�
�ʴ�Ϊ����3��
| ������� | ��ʢ�Լ������� | �Լ���װ�õ����� |
| A | ����ʯ��ˮ | |
| B | ||
| C | ����ȼ������ˮ�������ձ��ڱ�����ˮ����֣���֤��H2���� | |
| D | һ����̼ȼ�����ɶ�����̼��������̼��ʹ����ʯ��ˮ����ǣ���֤��CO���� |
������������Ҫ����ѧ��������ѧ֪ʶ�����ͽ��ʵ�������������������ѧ�����������˼ά��ȣ�ǿ����ѧ������֪ʶ��������

��ϰ��ϵ�д�
�����Ŀ